Salting preparation method of polypeptide
A salt-forming and refined peptide technology, which is applied in the field of peptide drug preparation, can solve problems such as complex operation procedures, long production cycle, and many impurities, and achieve high sample recovery rate, high sample quality, and stable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
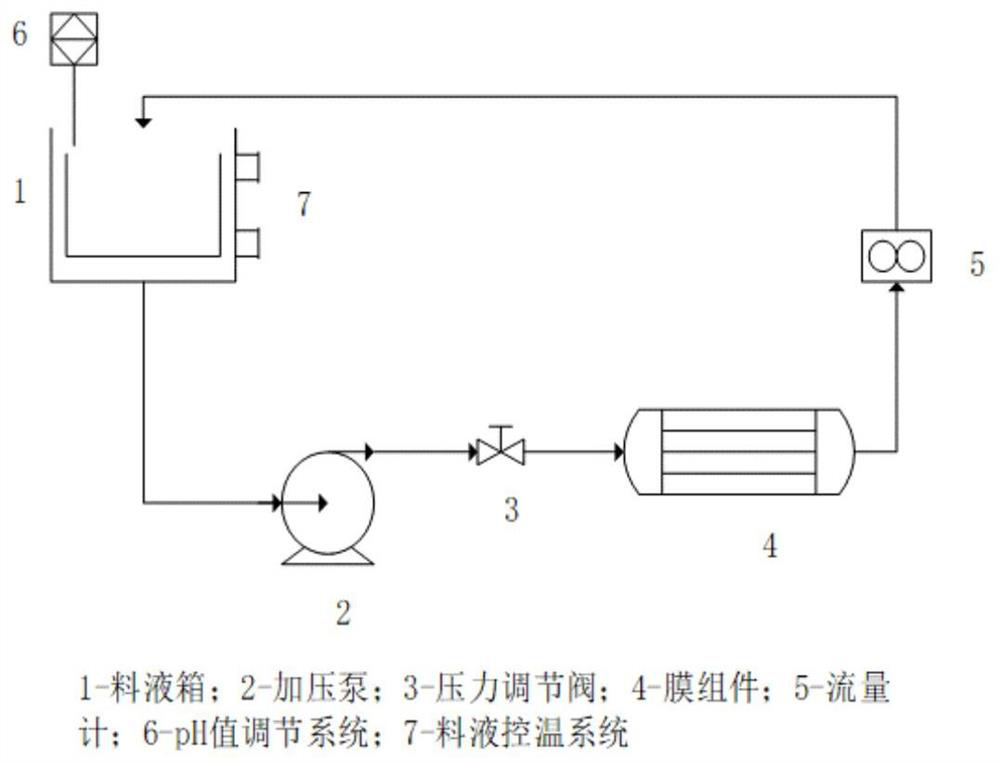
[0038] Embodiment 1: the application of nanofiltration salt-forming technology, the salt-forming method of pramlintide, the concrete steps are as follows:
[0039] 1) Pass the pramlintide stock solution through a 0.22 μm microporous membrane to remove insoluble impurities in the solution;
[0040] 2) Add the solution from which the insoluble impurities have been removed into the feed tank, add buffer salt, the buffer is 10mM potassium dihydrogen phosphate and adjust the pH to 7.5 with potassium hydroxide through the pH adjustment system; cool down to 5 through the feed liquid temperature control system °C;
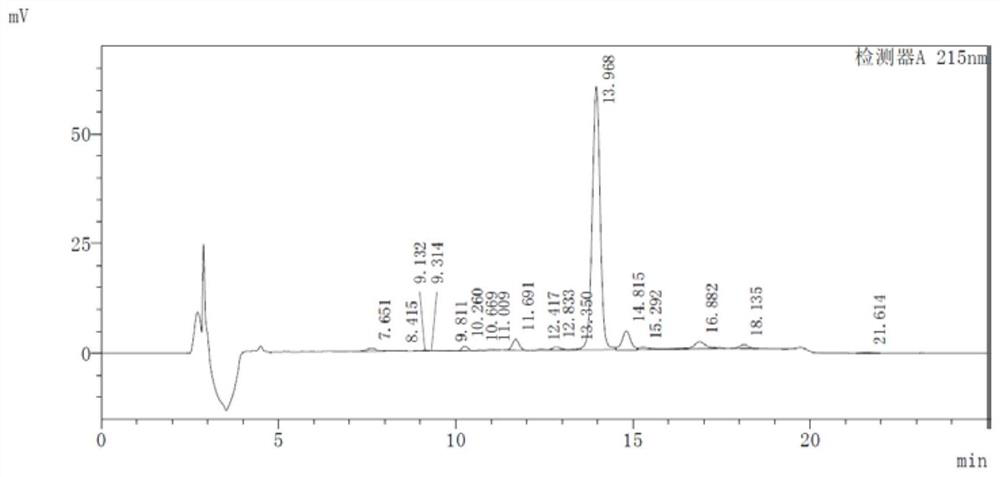
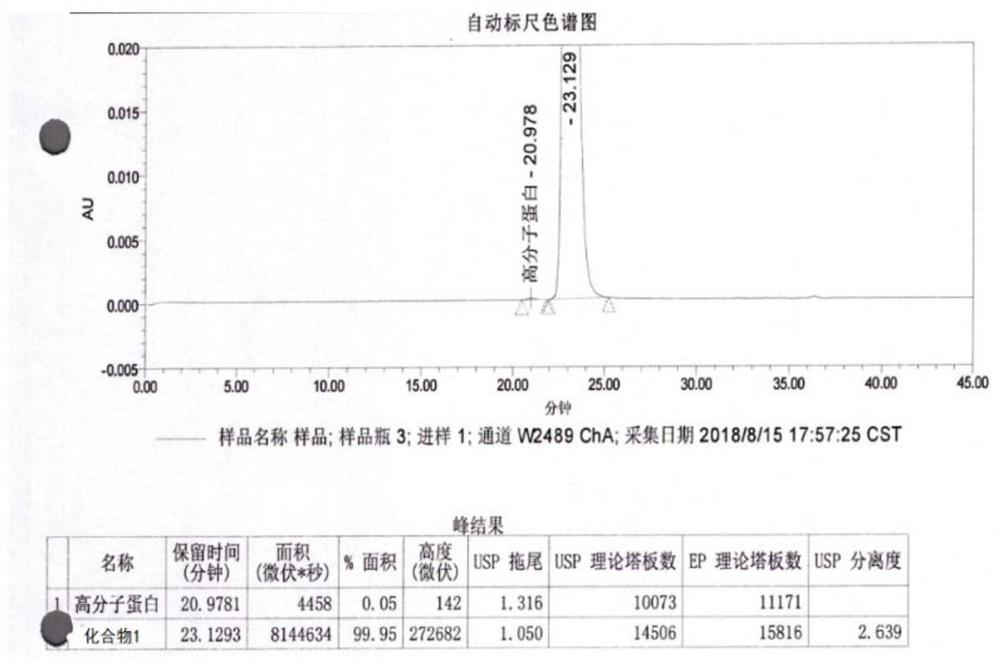
[0041] 3) The pressurizing pump transports the feed liquid to the membrane module for dialysis, salt exchange and concentration, the control pressure is 1.3Mpa, and the flow rate is 12L / hour. During the nanofiltration process, the buffer solution was continuously supplemented; the pramlintide sample solution containing potassium ions was obtained with a concentration of 3...
Embodiment 2
[0043] Embodiment 2: the application of nanofiltration salt-forming technology, the new salt-forming method of thymus method, the concrete steps are as follows:
[0044] 1) Pass the thymus method new mother liquor through a 0.22 μm microporous membrane to remove insoluble impurities in the solution;
[0045] 2) The solution from which insoluble impurities have been removed is put into the feed tank, and a buffer salt is added. The buffer is 50 mM ammonium bicarbonate and the pH is adjusted to 9.5 with ammonia water through the pH adjustment system; the temperature is lowered to 10° C. through the feed liquid temperature control system;
[0046]3) The pressurizing pump transports the feed liquid to the membrane module for salt exchange and concentration by dialysis, the control pressure is 1.5Mpa, and the flow rate is 16 liters / hour. During the nanofiltration process, the buffer solution was continuously supplemented; the thymus method new sample solution containing ammonium io...
Embodiment 3
[0048] Embodiment 3: apply nanofiltration salt-forming technology, the new salt-forming method of thymus method, concrete steps are as follows:
[0049] 1) Pass the thymus method new mother liquor through a 0.22 μm microporous membrane to remove insoluble impurities in the solution;
[0050] 2) The solution from which the insoluble impurities have been removed is put into the feed tank, buffer salt is added, the buffer is an ammonia solution with pH=9.5, and pH=9.5 is adjusted by the pH value control system; the temperature is lowered to 20°C by the feed liquid temperature control system;
[0051] 3) The pressurizing pump transports the feed liquid to the membrane module for dialysis, salt exchange and concentration, the control pressure is 1.3Mpa, and the flow rate is 12 liters / hour. During the nanofiltration process, the buffer solution was continuously supplemented; the thymus method new sample solution containing ammonium ions was obtained, and the concentration was 70 mg / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com