Synthesis method of o-tert-butyl aniline
A technology of o-tert-butylaniline and synthesis method, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of low reaction yield, cumbersome post-processing, high energy consumption, and achieve selectivity Good, good product quality, good yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
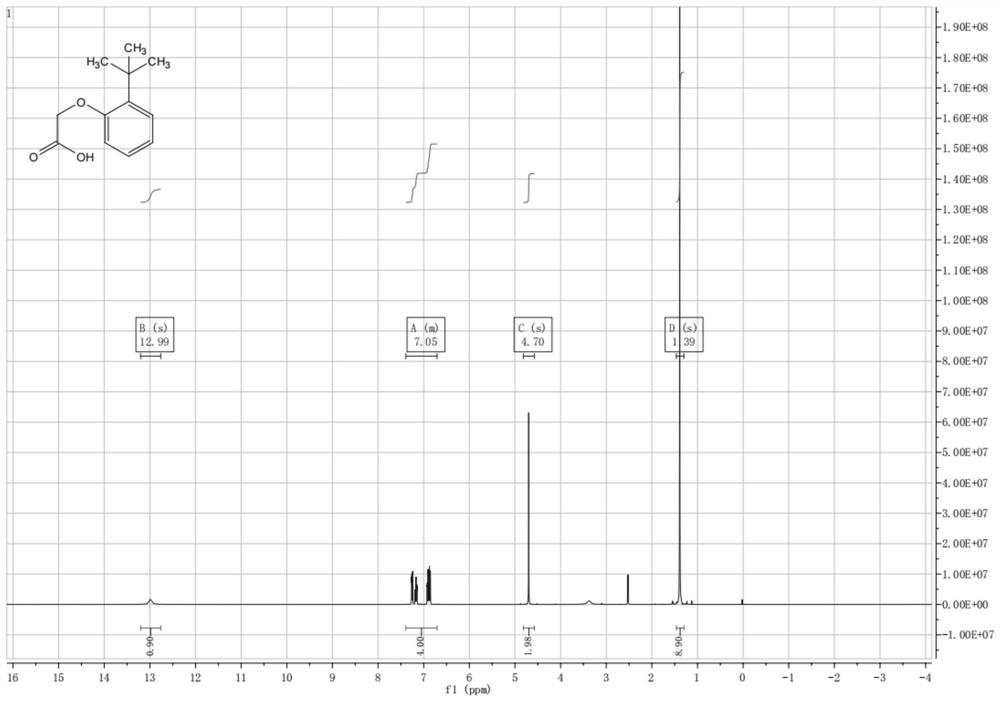
[0060] Example 1 Preparation of 2-(2-tert-butyl)phenoxy)acetic acid
[0061] 50.0 g of 2-tert-butylphenol and 500 mL of acetonitrile were added to a three-necked flask, 66.6 g of sodium hydroxide was added, the temperature was raised to 50° C., and stirring was started. 91.6g of 2-bromoacetic acid was added dropwise, the internal temperature was controlled to be 50±5°C, and the mixture was kept stirring for 4h. The reaction was monitored by TLC (petroleum ether:ethyl acetate=4:1) and the reaction was complete. Concentrate at 50 °C until no obvious droplets remain, add 500 mL of water and 350 mL of methyl tert-butyl ether, add 3N dilute hydrochloric acid to adjust pH=1~3, and use 350 mL of methyl tert-butyl ether for the aqueous phase after separation. Extracted, combined the organic phases and concentrated to dryness to obtain a light yellow solid, which was dried at 45°C to obtain 62.0 g, yield: 89.5%, see 1H NMR chart figure 1 shown.
Embodiment 2
[0062] Example 2 Preparation of 2-(2-tert-butyl)phenoxy)acetic acid
[0063] 25.0 g of 2-tert-butylphenol and 200 mL of methyl ethyl ketone were added to a three-necked flask, 33.3 g of sodium hydroxide was added, the temperature was raised to 55° C., and stirring was started. 45.8g of 2-bromoacetic acid was added dropwise, the internal temperature was controlled to be 50±5°C, and the mixture was kept stirring for 4h. The reaction was monitored by TLC (petroleum ether:ethyl acetate=4:1) and the reaction was complete. Concentrate at 50°C until no obvious droplets remain, add 250 mL of water and 170 mL of methyl tert-butyl ether, add 3N dilute hydrochloric acid to adjust pH=1~3, and use 150 mL of methyl tert-butyl ether for the aqueous phase after separation. Extracted, combined the organic phases and concentrated to dryness to obtain a light yellow solid, which was dried at 45°C to obtain 32.5 g, yield: 93.7%.
Embodiment 3
[0064] Example 3 Preparation of 2-(2-tert-butyl)phenoxy)acetic acid
[0065] 45.0 g of 2-tert-butylphenol and 500 mL of toluene were added to a three-necked flask, 66.6 g of sodium hydroxide was added, the temperature was raised to 70° C., and stirring was started. Add 82.5 g of 2-bromoacetic acid dropwise, control the internal temperature to be 50±5°C, and keep stirring for 5h. The reaction was monitored by TLC (petroleum ether:ethyl acetate=4:1) and the reaction was complete. 500 mL of water was added, and 3N dilute hydrochloric acid was added to adjust pH=1~3. After liquid separation, the organic phase was concentrated to dryness to obtain a pale yellow solid, which was dried at 45° C. to obtain 50.3 g, yield: 80.6%.
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com