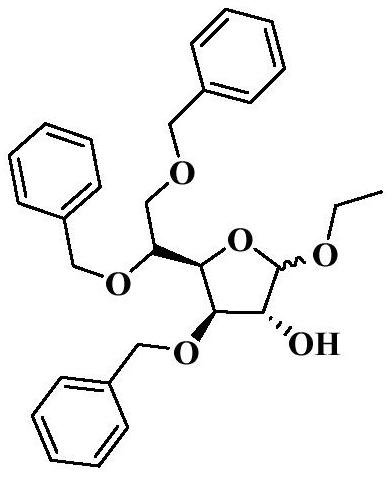
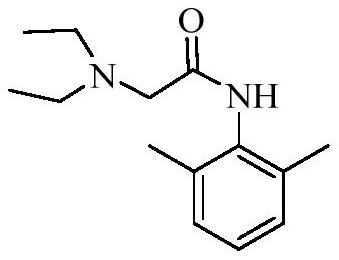
Tribenoside lidocaine suppository and preparation method thereof
A technology of tribenzyl glycoside lidocaine and lidocaine, which is applied in the directions of pharmaceutical formulations, suppository delivery, medical preparations of inactive ingredients, etc., and can solve the problems of instability, large amount of related substances in suppositories, poor stability of tribenzyl glycosides, etc. , to achieve the effect of small related substances, small voids in the tail, and moderate hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Preparation Process:
[0028] Completely melt SUPPOCIRE A and SUPPOCIRE NB at 60°C in advance, add lanolin, stir and mix to dissolve, after complete dissolution, continue to stir while cooling to 50°C, add lidocaine, and start stirring until lidocaine is completely dissolved. . Continue to cool down to 40°C while stirring, add the tribenzyl glucoside raw material, stir evenly, start filling into the double aluminum blister, and perform gradient cooling in the freezer, that is, first cool down to 15-25°C, cool for 10- After 15 minutes, the temperature is lowered to 0 to 5°C, cooled for 15 to 20 minutes, and then sealed.
Embodiment 2
[0030]
[0031] Preparation Process:
[0032] Completely melt SUPPOCIRE A and SUPPOCIRE NB at 60°C in advance, add lanolin, stir and mix to dissolve, after complete dissolution, continue to stir while cooling to 50°C, add lidocaine, and start stirring until lidocaine is completely dissolved. . Continue to cool down to 40°C while stirring, add the tribenzyl glucoside raw material, stir evenly, start filling into the double aluminum blister, and perform gradient cooling in the freezer, that is, first cool down to 15-25°C, cool for 10- After 15 minutes, the temperature is lowered to 0 to 5°C, cooled for 15 to 20 minutes, and then sealed.
Embodiment 3
[0034]
[0035]
[0036] Preparation Process:
[0037] Completely melt SUPPOCIRE A and SUPPOCIRE NB at 60°C in advance, add lanolin, stir and mix to dissolve, after complete dissolution, continue to stir while cooling to 50°C, add lidocaine, and start stirring until lidocaine is completely dissolved. . Continue to cool down to 40°C while stirring, add the tribenzyl glucoside raw material, stir evenly, start filling into the double aluminum blister, and perform gradient cooling in the freezer, that is, first cool down to 15-25°C, cool for 10- After 15 minutes, the temperature is lowered to 0 to 5°C, cooled for 15 to 20 minutes, and then sealed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com