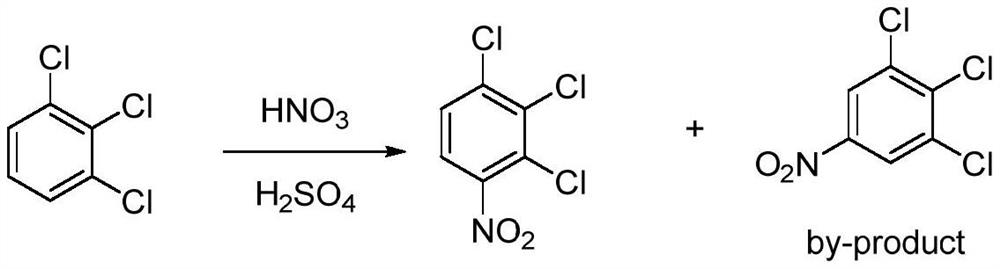
Method for preparing 2, 3, 4-trichloronitrobenzene through micro-channel nitration reaction
A technology of trichloronitrobenzene and nitration reaction, which is applied in the field of preparation of pesticide chemical intermediates, can solve the problems of large impurity ratio, difficult separation, poor selectivity, low production efficiency, etc., and achieves reduction of post-processing process and product purity High and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Mixed acid preparation: Mix 98% sulfuric acid and 95% nitric acid to obtain mixed acid, wherein the molar ratio of nitric acid and sulfuric acid is 1:4.0.
[0028] Preparation of raw materials: preheat and dissolve 1,2,3-trichlorobenzene without adding solvent 1,2-dichloroethane, in which the moles of 1,2,3-trichlorobenzene and 1,2-dichloroethane are The ratio was 1:0; no catalyst was added.
[0029] The temperature of the external heat exchanger was set to 70°C, that is, the preheating temperature was set to 70°C, so that the system circulation temperature reached equilibrium.
[0030] The 1,2,3-trichlorobenzene and mixed acid are respectively passed into the preheating module through a tetrafluoro advective pump for preheating, and then enter the reaction module of the microchannel continuous flow reactor. Adjust the flow rate of 1,2,3-trichlorobenzene to 4.2mL / min, adjust the flow rate of mixed acid to be 12.1mL / min, make the molar ratio of 1,2,3-trichlorobenzene an...
Embodiment 2
[0034] Mixed acid preparation: Mix 98% sulfuric acid and 95% nitric acid to obtain mixed acid, wherein the molar ratio of nitric acid and sulfuric acid is 1:1.08.
[0035] Preparation of catalyst caprolactam-m-hydroxybenzenesulfonate ionic liquid: add 0.2 moles of caprolactam and 40 mL of water to a round-bottomed flask to fully dissolve, and add 40 mL of m-hydroxybenzenesulfonic acid aqueous solution with a concentration of 5 mol / L dropwise in an ice-water bath with stirring, The reaction was carried out at room temperature for 18h. Rotary evaporation, washing with benzene, and vacuum drying at 65 °C to obtain caprolactam-m-hydroxybenzenesulfonate ionic liquid.
[0036] Preparation of raw materials: 1,2,3-trichlorobenzene and 1,2-dichloroethane, a solvent of equal quality, are prepared into a mixture, wherein the mixture of 1,2,3-trichlorobenzene and 1,2-dichloroethane is The molar ratio is 1:1.83; and the caprolactam-m-hydroxybenzenesulfonate ionic liquid is added therein, wh...
Embodiment 3
[0042] Mixed acid preparation: Mix 98% sulfuric acid and 95% nitric acid to obtain mixed acid, wherein the molar ratio of nitric acid and sulfuric acid is 1:1.08.
[0043] Preparation of catalyst caprolactam p-hydroxybenzenesulfonate ionic liquid: add 0.2 moles of caprolactam and 40 mL of water to a round-bottomed flask to fully dissolve, and add 40 mL of an aqueous solution of p-hydroxybenzenesulfonic acid with a concentration of 5 mol / L dropwise in an ice-water bath with stirring, The reaction was carried out at room temperature for 18h. Rotary evaporation, washing with benzene, and vacuum drying at 65 °C to obtain caprolactam p-hydroxybenzenesulfonate ionic liquid.
[0044] Preparation of raw materials: 1,2,3-trichlorobenzene and 1,2-dichloroethane, a solvent of equal quality, are prepared into a mixture, wherein the mixture of 1,2,3-trichlorobenzene and 1,2-dichloroethane is The molar ratio was 1:1.83; and the caprolactam-p-hydroxybenzenesulfonate ionic liquid was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com