Pullulanase mutant, engineering bacterium and application of pullulanase mutant
A technology of pullulanase and pullulanase protein is applied in the field of preparing pullulanase with improved thermal stability and acid resistance, which can solve the problems of poor stability and low catalytic activity, and achieve good catalytic performance and industrial properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Construction of wild-type pullulanase recombinant bacteria
[0024] According to the gene (KY273924) of type I pullulanase derived from thermoanaerobic bacillus (Anoxybacillus sp.) AR-29 in Genbank, the cloning primers of pullulanase were designed as shown in Table 1, and the Fragment and inserted into the vector pET-32a(+) between EcoR V and Xho I restriction site to obtain recombinant plasmid pET-32a(+)-PulAR (nucleotide sequence shown in SEQ ID NO.2, encoding The amino acid sequence of the protein is shown in SEQ ID NO.1), the recombinant plasmid pET-32a(+)-PulAR with correct sequencing was transformed into E.coli BL21(DE3), and a single colony was picked for sequencing to obtain recombinant bacteria E. coli BL21(DE3) / pET-32a(+)-PulAR, namely wild-type E. coli BL21(DE3) / pET-32a(+)-PulAR.
[0025] SEQ ID NO.1
[0026] MYEVFSSLILKTNEKMGLFILGGANLLTVHRTFEAYLDTMTVITILIPKSYHSGMVGNFIIEKPNGERCQLQVAKREDLWTSIKYECVIDFAVEIGRRYLIYDDHGAFTDLQIGAVIRTAEFDEQFYYEGNDLGITYTP...
Embodiment 2
[0029] Example 2: Construction and screening of pullulanase mutants
[0030] 1. Construction of pullulanase mutants
[0031] According to the gene sequence of pullulanase PulAR shown in SEQ ID NO.2, design and synthesize mutant primers (Table 1) introducing mutations Q355, A365, T399, V401, Y491, H499 and T504, using the obtained in Example 1 The recombinant plasmid pET-32a(+)-pulAR was used as the template, the whole plasmid was amplified by PCR, transformed into E. coli E.coli BL21(DE3), sequenced and identified, and the single mutant E.coli BL21(DE3)-PulAR was obtained -Q355H, E.coli BL21(DE3)-PulAR-A365V, E.coli BL21(DE3)-PulAR-T399S, E.coli BL21(DE3)-PulAR-V401T, E.coli BL21(DE3)-PulAR-V401C , E.coli BL21(DE3)-PulAR-Y491V, E.coli BL21(DE3)-PulAR-H499A and E.coliBL21(DE3)-PulAR-T504V.
[0032] PCR amplification system: 1 μL forward primer (100 μM), 1 μL reverse primer (100 μM), 12.5 μL 2× Phanta buffer, 0.5 μL dNTP mix (10 mM each), 1 μL plasmid template, 0.5 μL DNA poly...
Embodiment 3
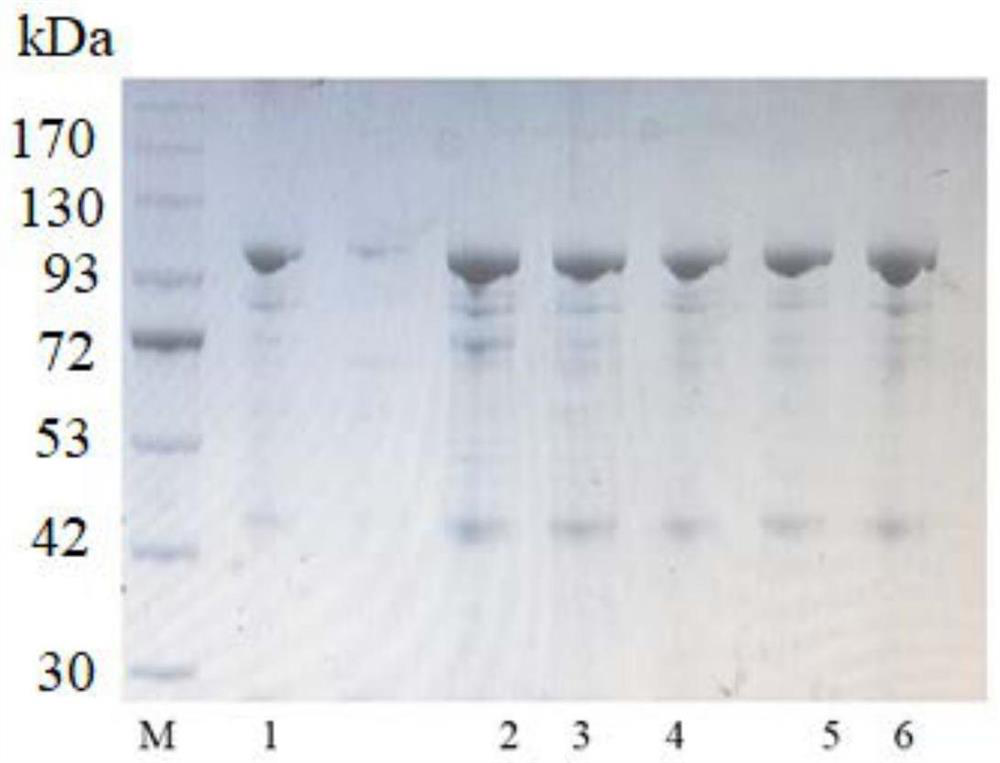
[0046] Example 3: Combinatorial Mutation and Purification of Pullulanase Protein
[0047] 1. Combination mutation
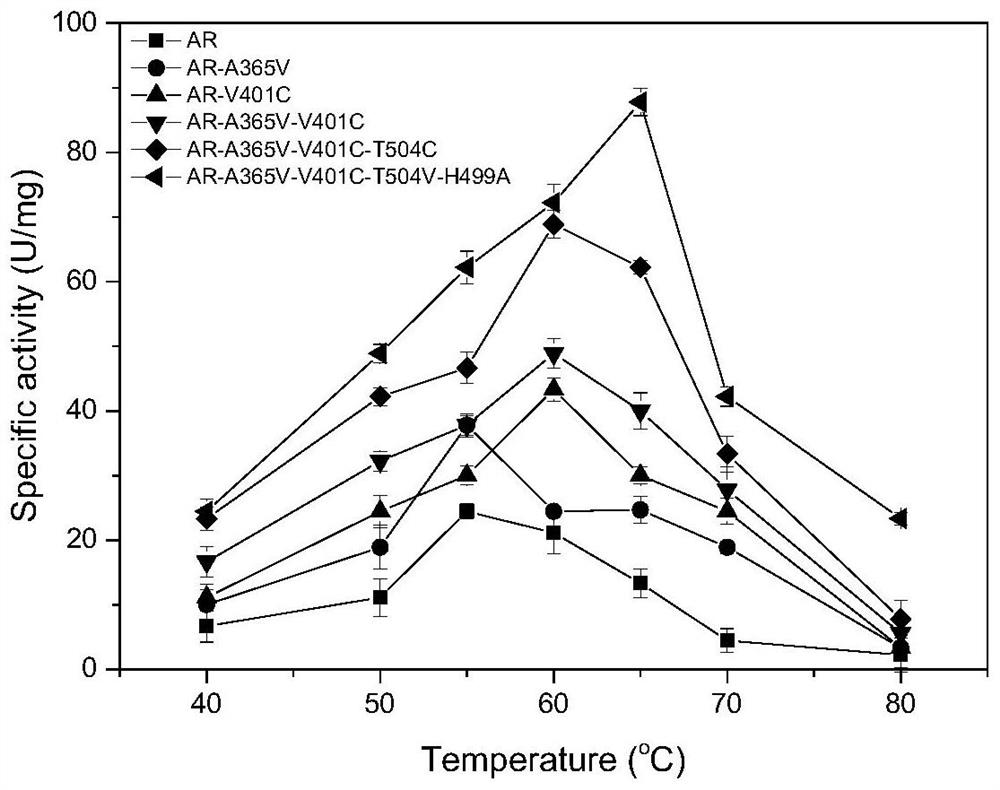
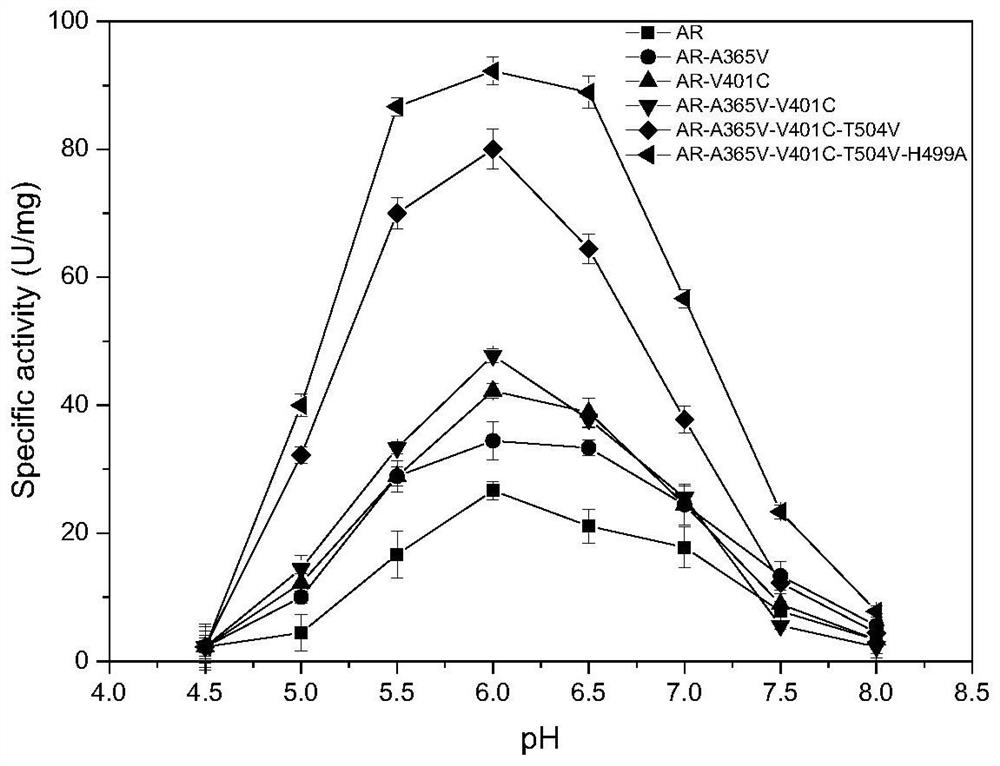
[0048] The beneficial mutations A365V, V401C, T504V and H499A of the pullulanase obtained in Example 2 were subjected to combined mutation research, and combined mutants PulAR-A365V / V401C, PulAR-A365V / V401C / T504V, PulAR-A365V / V401C / T504V / H499A, as follows:
[0049] (1) Construction of combined mutant PulAR-A365V / V401C
[0050] Using the plasmid pET-32a-PulAR-A365V of the single mutant E.coli BL21(DE3)-PulAR-A365V of Example 2 as a template, and using Q355H(F) and Q355H(R) in Table 2 as primers, Example 2 was used Methods Whole plasmid PCR was performed, and the mutant PulAR-A365V / V401C was obtained by screening.
[0051] (2) Construction of combined mutant PulAR-A365V / V401C / T504V
[0052] Taking the above-mentioned plasmid pET-32a-PulAR-A365V / V401C of the constructed combined mutant E.coli BL21(DE3)-PulAR-A365V / V401C as a template, and taking V401C(F) and V4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com