Preparation method and application of halophilic archaea extracellular protease truncation
A technology of extracellular protease and halophilic archaea, applied in the field of genetic engineering, can solve the problems of separation and purification limitations, poor stability, depolymerization or inactivation of protease, etc., and achieve simple operation, excellent enzymatic characteristics, and resistance receptivity outstanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) The recombinant plasmid pET28a-hlyA was transformed into a prokaryotic host, and expression was induced;
[0058] The recombinant plasmid pET28a-hlyA was transformed into E.coli BL21 expression strain to construct an expression recombinant strain. Then transfer 2 mL of the recombinant expression bacterial solution to 200 mL of LB liquid medium containing 50 μg / mL kanamycin, and shake at 37 °C to grow to the logarithmic phase (OD). 600 Between 0.55 and 0.65), IPTG with a final concentration of 0.5 mM was added to induce expression for 4 to 5 h; the cells were collected by centrifugation at 4°C.
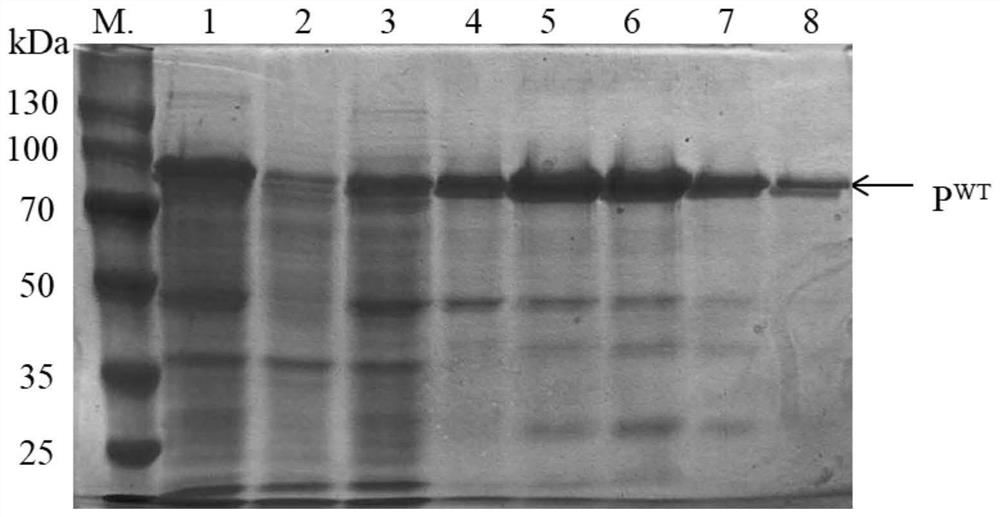
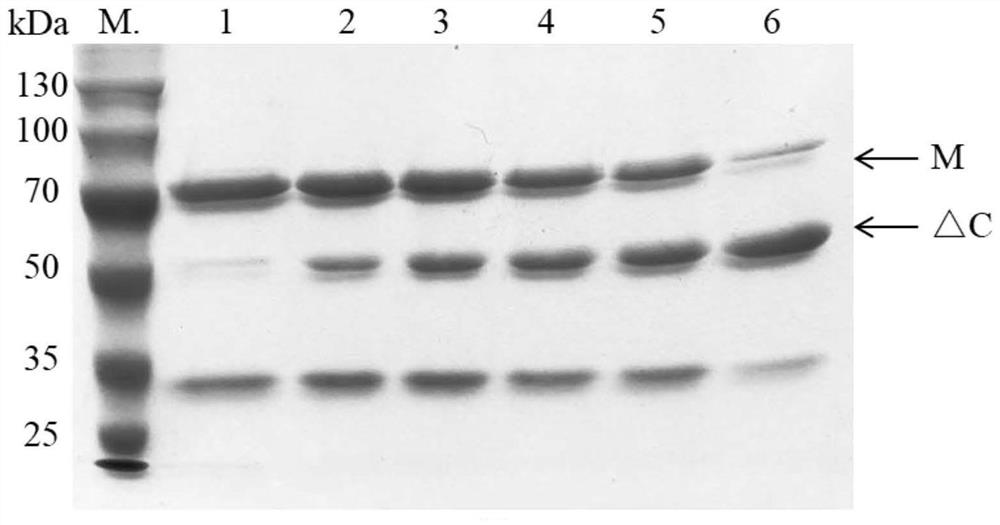
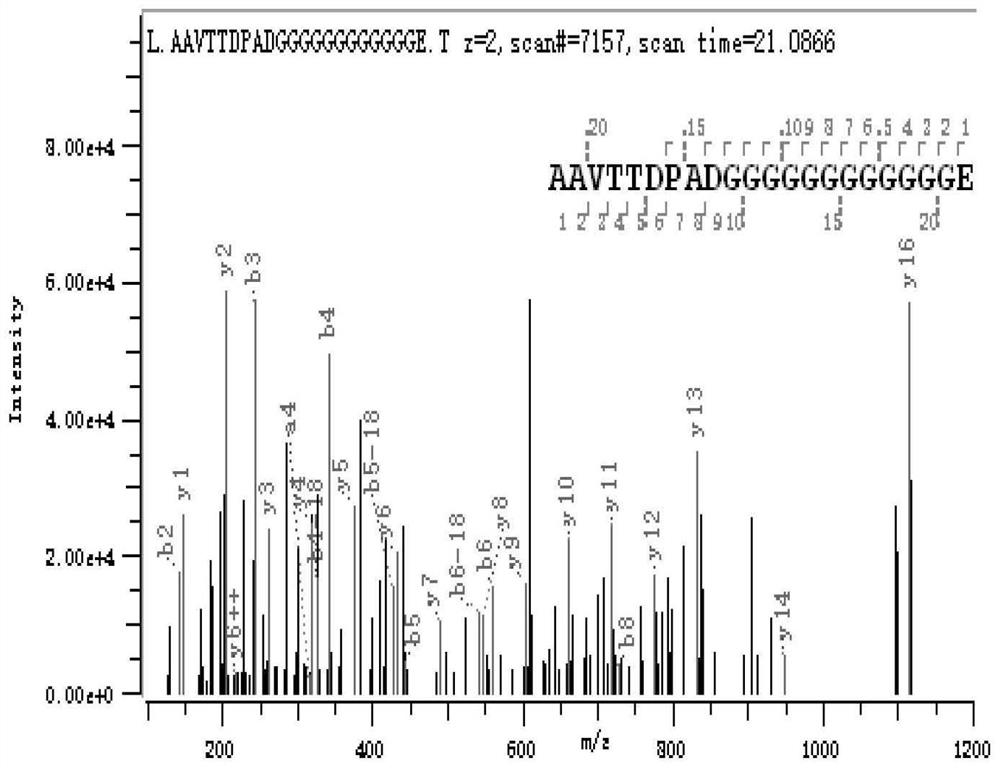
[0059] (2) Purification of halophilic archaeal protease HlyA:
[0060] Collect the cells after induction and expression in step (1) (E.coli BL21 cells expressing protease HlyA) in cell lysate (8M urea, 10mM CaCl) 2 , 50 mM Tris-HCl, pH 8.0), resuspended in 50 mM Tris-HCl, pH 8.0), and then sonicated for 3 s at 5 s intervals until the cells were translucent. After centrifug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com