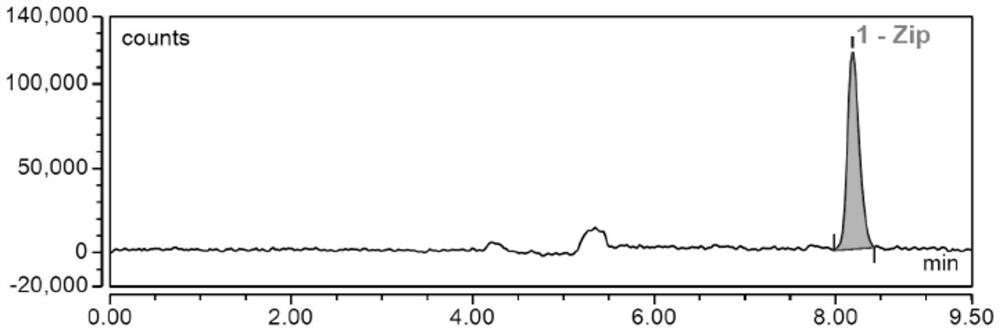
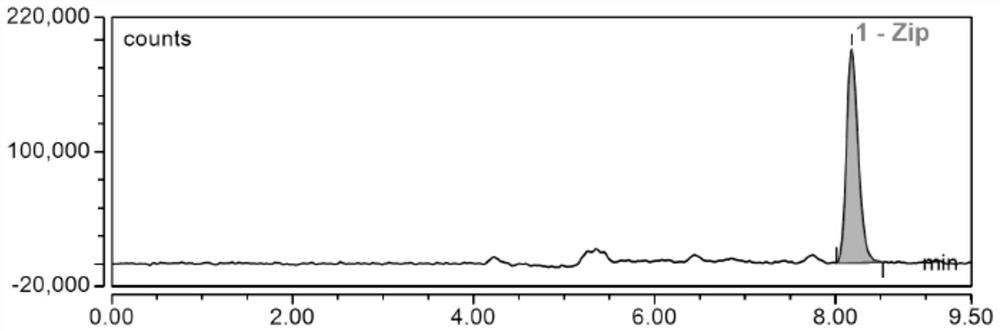
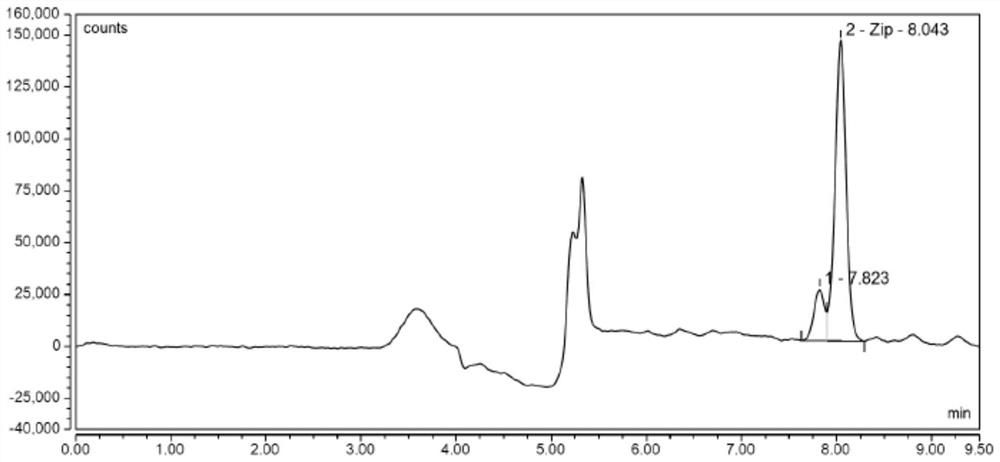
Liquid chromatographic analysis method for detecting ziprasidone in blood
A technology for liquid chromatography analysis and ziprasidone, which is applied in the field of liquid chromatography analysis for detecting ziprasidone in blood, can solve the problems of large sample volume demand, long analysis time and high analysis cost, and achieves good sensitivity and Accuracy, simplification of preprocessing steps, the effect of shortening analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] The present embodiment is used to illustrate the specific detection steps of the liquid chromatographic analysis method for detecting ziprasidone in blood:
[0123] 1. Calibration of standard solutions
[0124] 1.1 Preparation of standard working solution:
[0125] Accurately weigh 2 mg of ziprasidone standard, put it in a 2 mL cryovial, add 2 mL of anhydrous methanol, and obtain standard stock solution A;
[0126] Take an appropriate amount of standard stock solution A, dilute it with 50v / v% methanol aqueous solution, the concentration of ziprasidone in the obtained standard working solution is 6400ng / mL, and then continue to dilute with 50v / v% methanol aqueous solution to prepare the concentration ziprasidone solutions of 200ng / mL, 400ng / mL, 600ng / mL, 800ng / mL, 1600ng / mL, 3200ng / mL, and 6400ng / mL, respectively, and stored at -80°C;
[0127] 1.2 Obtain the standard curve:
[0128] Firstly, 10μL of standard working solution and 90μL of blank serum were respectively t...
Embodiment 2
[0157] This example is used to illustrate the linear relationship and the limit of quantification of the method of the embodiment of the present invention:
[0158] Add 10 μL of the ziprasidone standard working solution of each concentration prepared above, add 90 μL of blank serum and mix for 1 min, add 300 μL of acetonitrile, vortex and mix for 3 min at 2000 rpm, centrifuge at 14000 r / min for 10 min, and draw 350 μL of the supernatant was injected and analyzed, and the concentration of ziprasidone was in the range of 20ng / mL to 640ng / mL. According to the measurement conditions of this example, the concentration was measured from low to high, and the quantitative chromatographic peak area-concentration was plotted , obtain the standard curve; prepare a series of low-concentration standard solutions, add 10 μL of ziprasidone standard solutions of various concentrations prepared above, add 90 μL of blank serum or plasma respectively, and mix well, after pretreatment, injection a...
Embodiment 3
[0163] This example is used to illustrate the recovery rate and precision of the method of the embodiment of the present invention.
[0164] The standard working solution of ziprasidone was prepared into three concentrations of low, medium and high for sample addition recovery experiment and precision experiment, and the measurement was carried out according to the method of this example. The analysis and determination were repeated for 3 batches, and ziprasidone was recovered The rate and precision are shown in Table 2, respectively. The average recoveries of ziprasidone in the low, medium and high addition levels ranged from 99.0% to 101.3%, and the relative standard deviations were 0.83% to 1.96%. The results are shown in Table 2.
[0165] Table 2 Ziprasidone spiked recovery and precision
[0166] Scalar 40ng / mL 80ng / mL 320ng / mL The average recovery rate 100.5% 101.3% 99.0% Precision RSD 6.76% 1.20% 1.40%
[0167] Based on the above ver...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com