Metal zinc negative electrode protected by chemical passivation layer as well as preparation method and application of metal zinc negative electrode
A technology of chemical passivation and metal zinc, which is applied in the direction of electrode manufacturing, electrochemical generator, metal material coating process, etc., can solve the problems of irreversible consumption of zinc, lower deposition/dissolution efficiency, lower battery life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) A commercial zinc foil (10×10 cm, 100 μm) was ultrasonically cleaned with deionized water and absolute ethanol for 15 minutes in sequence, and then placed in a vacuum drying box for vacuum drying at 60°C after cleaning.
[0028] 2) Weigh 10g NaSiO 3 , add 500 mL of deionized water, stir in a water bath for 30 min at a temperature of 40 °C to fully dissolve it, and add 1 mol L of water dropwise. -1 HNO 3 Adjust the pH value of the passivation solution to make the pH value 3;
[0029] 3) Put the dried zinc foil in the passivation solution, keep the temperature of the water bath at 40°C, take it out after soaking for 5 minutes, wash the surface of the zinc foil with deionized water and absolute ethanol respectively, and place it in a vacuum drying oven after cleaning. Dry under vacuum at 60°C.
[0030] 4) The modified zinc foil obtained by drying is punched into an electrode disk with a diameter of 12 mm on a microtome, and a zinc|zinc symmetrical battery is assemble...
Embodiment 2
[0034] The rest of the conditions are the same as in Example 1, except that the soaking time of the zinc foil is changed to 15 min.
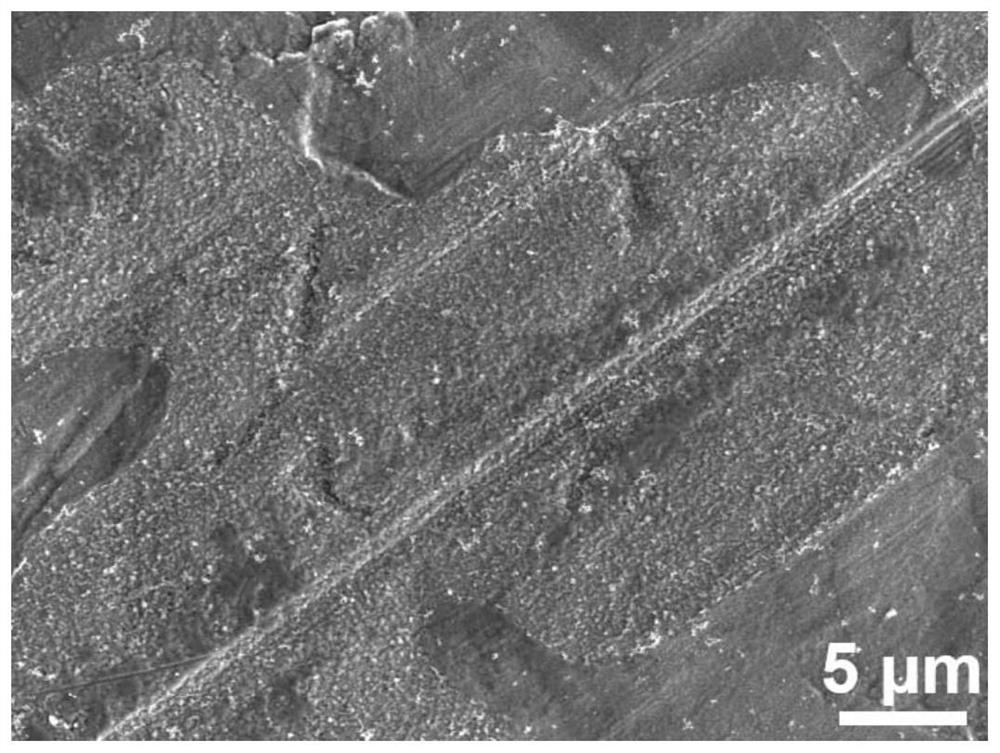
[0035] like figure 2 As shown in the figure, with the prolongation of the soaking time to a certain extent, the modified zinc negative electrode obtained in this example, the passivation layer becomes continuous and uniform, and completely covers the zinc substrate.
[0036] like Figure 5 As shown, due to the improvement of film formation quality, the modified zinc anode obtained in this example can run stably for 1100 h.
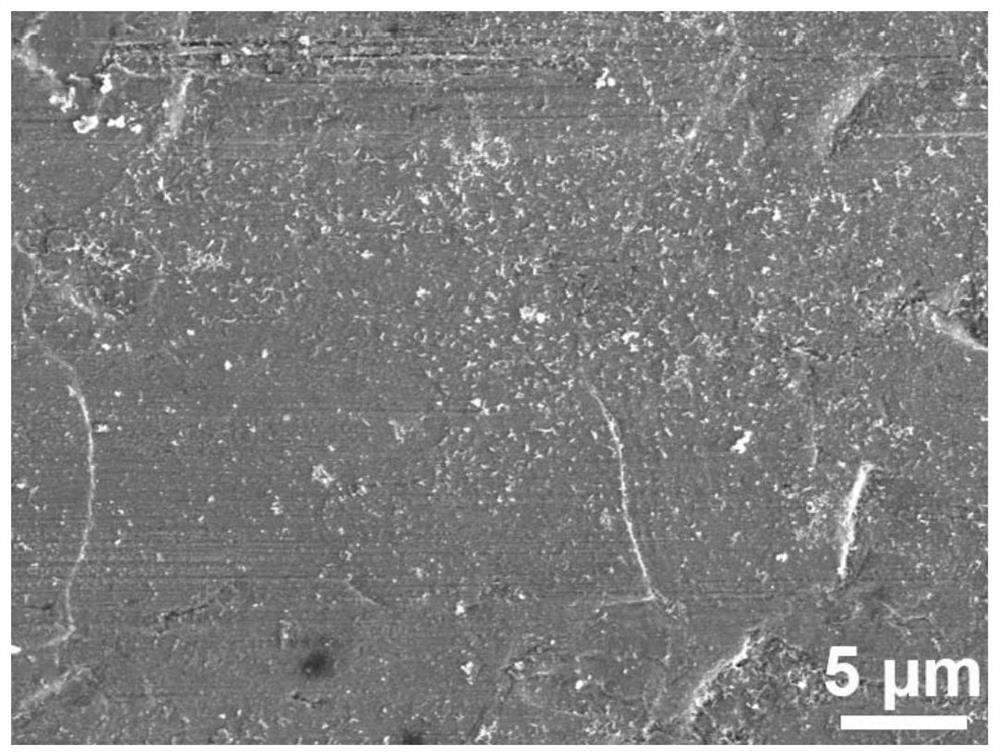
[0037] like Figure 7 and Figure 8 It can be seen from the comparison between the SEM image of the modified zinc anode after cycling in this example and the SEM image of the ordinary zinc anode after cycling that, compared with the ordinary zinc anode, due to the improvement of the film quality, the zinc anode after cycling has no dendrites. , exhibiting a flat, compact surface.
[0038] like Figure 9 As shown, due ...
Embodiment 3
[0040] The rest of the conditions are the same as in Example 1, except that the soaking time of the zinc foil is changed to 30 min.
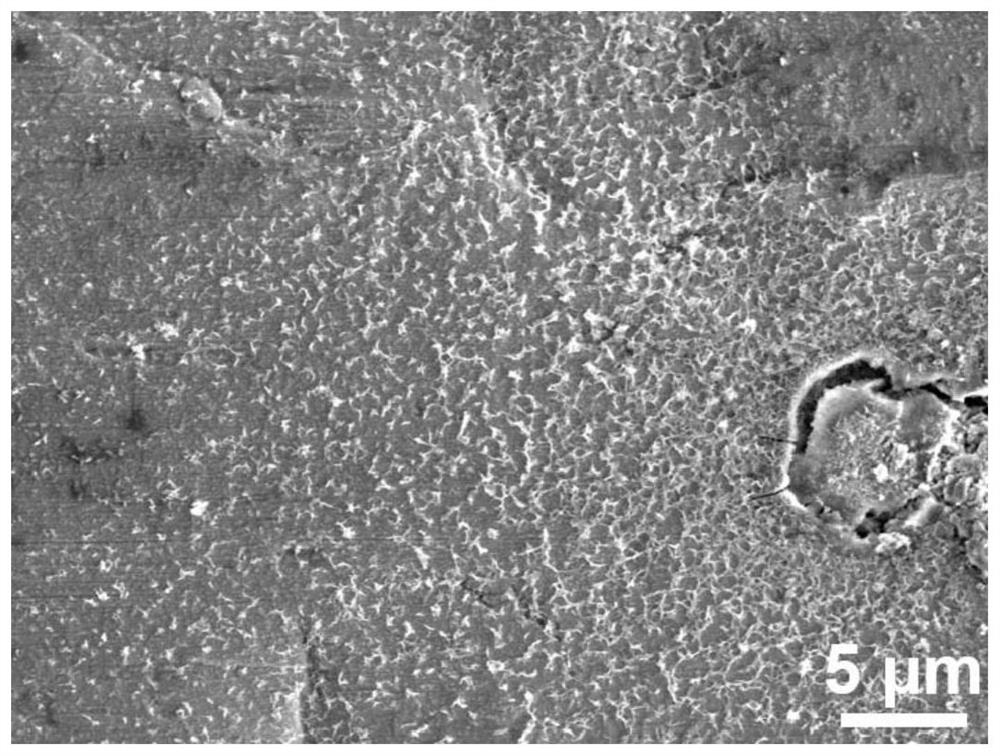
[0041] like image 3 As shown in the figure, due to the excessively long film formation time of the modified zinc negative electrode obtained in this example, part of the passivation layer begins to accumulate and fall off, exposing the underlying zinc substrate.
[0042] like Image 6 As shown, due to the detachment of the passivation layer, the cycle life of the modified zinc anode obtained in this example was reduced, and a short circuit occurred after 400 hours of operation.
[0043] To sum up, compared with the prior art, the beneficial effects of the present invention are:
[0044] (1) The present invention constructs a layer of chemical passivation layer on the surface of the metal zinc negative electrode in situ, with uniform thickness and continuous film layer, which can effectively coat the zinc negative electrode, isolate water and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com