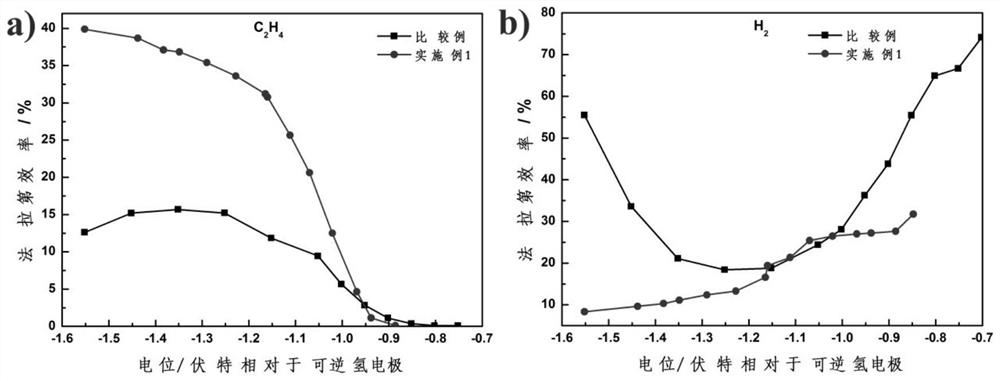
a co 2 Electrode for electrochemical reduction and its preparation and application
An electrochemical and electrode technology, applied in the field of electrochemical reduction of carbon dioxide, can solve the problems of small electrode reaction area, complex reaction process, low conversion rate, etc., and achieve the effect of high surface roughness and large electrochemical reaction area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
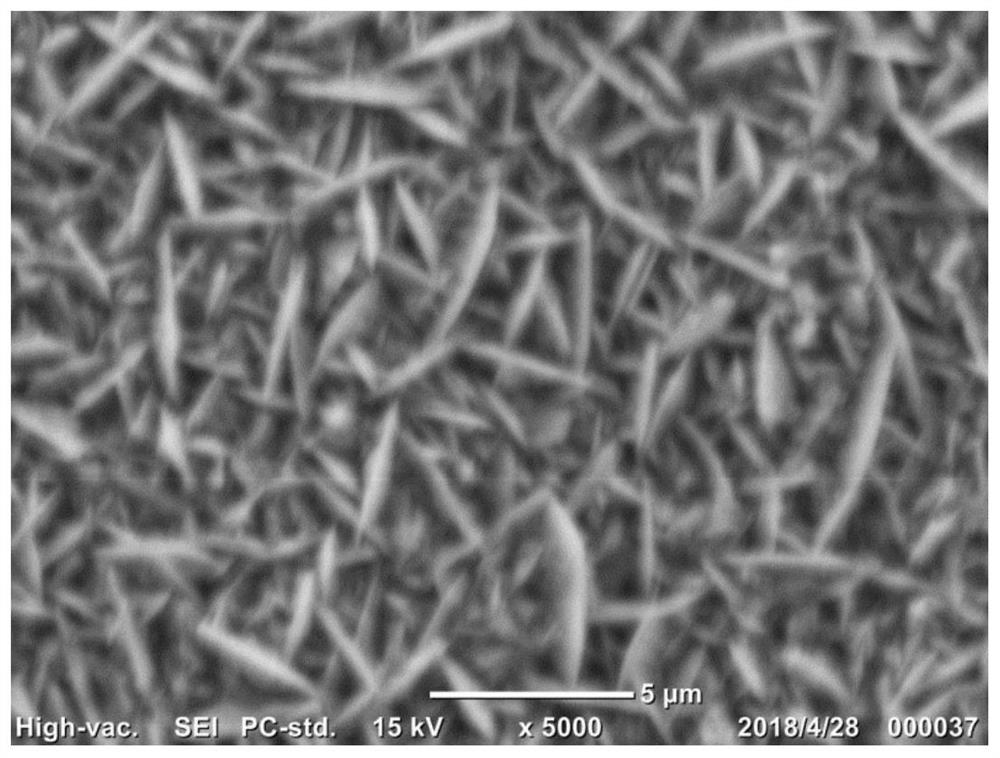
Embodiment 1
[0031] 1. Electrode material pretreatment: copper content ≥ 99.5%, thickness 100 microns, area 10 cm 2 The copper sheet is used as the electrode material, firstly after 1200 # After the water abrasive paper is polished smooth, soak it in concentrated hydrochloric acid with a volume fraction of 36% to 38% at room temperature for 20 minutes to remove surface impurities, then rinse it with a large amount of deionized water until it is neutral, and blow it with high-purity nitrogen. Dry;
[0032]2. Electroplating solution preparation: to analyze pure grade CuSO 4 ·5H 2 O is used as the main salt, and ethylenediaminetetraacetic acid (EDTA) and polyvinylpyrrolidone (PVP k12, average molecular weight 3000) are used as the first and second additives respectively, and 250ml of electroplating solution is prepared with ultrapure water with a resistivity of 18.2MΩ. Control the concentrations of the main salt, EDTA and PVP in the plating solution to be 0.1M, 1.5mM and 100ppm respectivel...
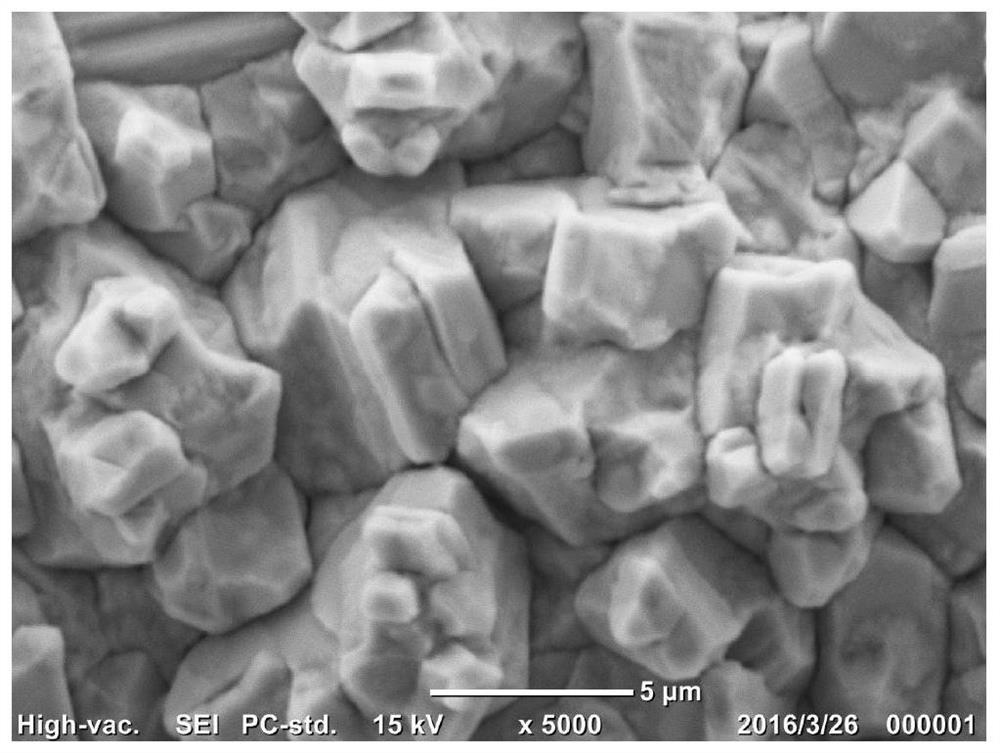
Embodiment 2
[0043] 1. Substrate material pretreatment: with a thickness of 200 microns, a porosity of 78%, and an area of 10 cm 2 The THP-H-060 carbon paper used as the electrode material was soaked in acetone solution for 20 minutes to remove the grease on the surface of the fiber, and then dried naturally after taking it out;
[0044] 2. Electroplating solution preparation: to analyze pure grade CuNO 3 ·3H 2 O is used as the main salt, ethylenediaminetetramethylene sodium phosphate (EDTMPS) and polyethylene glycol (PEG 4000) are used as the first and second additives respectively, and 250ml of electroplating solution is prepared with ultrapure water with a resistivity of 18.2MΩ. Control the concentration of main salt, EDTMPS and PVP in the plating solution to be 0.05M, 0.5mM and 20ppm respectively, with 6MHNO 3 Adjust the pH of the plating solution to 3.0, and prepare 100ml of the plating solution without adding the two additives at the same time, CuNO 3 The concentration is 0.05M,...
Embodiment 3
[0050] 1. Electrode material pretreatment: copper content ≥ 99.5%, thickness 100 microns, area 10 cm 2 The copper sheet is used as the electrode material, firstly after 1200 # After the water abrasive paper is polished smooth, soak it in concentrated hydrochloric acid with a volume fraction of 36% to 38% at room temperature for 20 minutes to remove surface impurities, then rinse it with a large amount of deionized water until it is neutral, and blow it with high-purity nitrogen. Dry;
[0051] 2. Electroplating solution preparation: to analyze pure grade CuCl·2H 2 O was used as the main salt, sodium alginate and polyethylene glycol (PEG 6000) with a relative molecular mass of 6000 were used as the first and second additives, and 250ml of electroplating solution was prepared with ultrapure water with a resistivity of 18.2MΩ. The concentrations of the main salt, sodium alginate and PEG6000 in the solution were 0.5M, 5mM and 500ppm respectively, the pH of the plating solution wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com