Proline racemase as well as preparation and application thereof
A racemase and proline technology, applied in the field of genetic engineering, can solve problems such as difficult access, small catalytic sites, and increased difficulty in inhibitor screening and design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0037] Experimental Example 1 Acquisition of proline racemase gene NParc2
[0038] (1) Screening of gibbon fecal microbial metagenomic proline racemase genes
[0039] From the constructed gibbon fecal microbial library, according to the analysis results of gene prediction, functional annotation and secreted protein prediction, the gene with the annotation result of proline racemase was screened, and the proline racemase gene NCPrac2 was obtained. The gene sequence is as shown in SEQ ID NO.1.
[0040] (2) Cloning of proline racemase gene NCPrac2
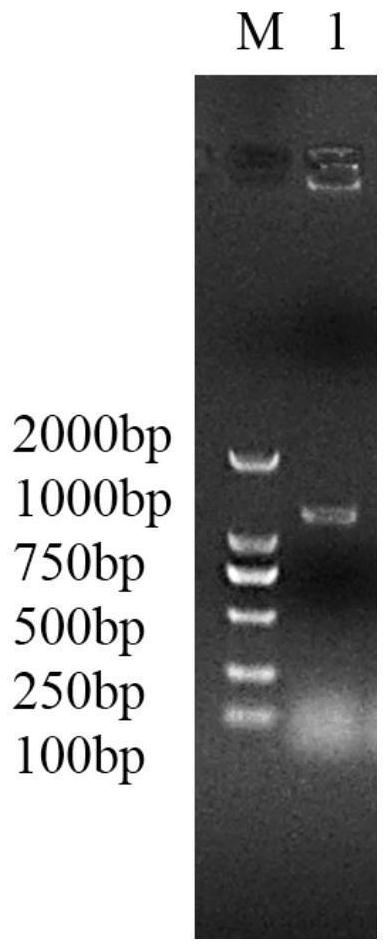
[0041] PCR amplification was performed using NCPrac2-F / NCPrac2-R as primer pair and Western black crested gibbon fecal microbial metagenomic DNA as template.
[0042] PCR reaction system (20.0 μL): metagenomic DNA 0.5 μL, PrimeSTAR Max 10 μL, NCPrac2-F 0.5 μL, NCPrac2-R 0.5 μL, ddH 2 O make up 20.0 μL.
[0043] PCR reaction parameters were: 98°C, 10s; 55°C, 15s; 72°C, 90s; 30 cycles; 72°C, 10 min; 4°C, 10 min.
[0044] PCR results ...
experiment example 2
[0050] Experimental Example 2 Preparation of proline racemase NCPRAC2
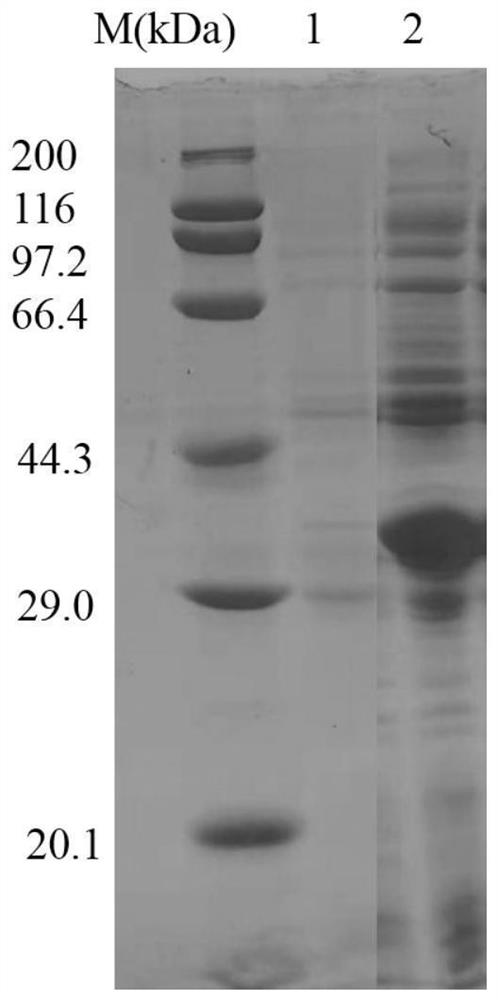
[0051] The proline racemase gene NCPrac2 prepared in Example 1 was connected with the plasmid pEASY-E2 to obtain a recombinant expression vector pEASY-E2-NCPrac2, and then transformed into E. coli BL21(DE3) to obtain a recombinant E. coli strain BL21(DE3) / NCPrac2. Take the E. coli strain BL21(DE3) / NCPrac2 containing the recombinant expression vector pEASY-E2-NCPrac2, inoculate it with 0.1% inoculum in LB (containing 100 μg / mL Amp) medium, and cultivate overnight at 37°C and 180rpm. Then this activated bacterial solution was inoculated into fresh LB (containing 100 μg / mL Amp) medium with 1% inoculum, and cultured at 37°C and 180 rpm for about 5-6 h (OD). 600 After reaching 0.8-1.0), IPTG with a final concentration of 0.7 mmol / L was added for induction, and cultured at 16 °C and 180 rpm for about 16 h. The cells were collected by centrifugation at 5000 rpm for 10 min. After suspending the cells with an app...
experiment example 3
[0053] Experimental Example 3 Determination of Properties of Proline Racemase NCPRAC2
[0054] The enzymatic activity of the recombinant proline racemase NCPRAC2 was determined by a two-step method of racemization and oxidation.
[0055] One unit of enzyme activity (U) was defined as the amount of enzyme required to catalyze the production of 1 μmol of D-Pro within 1 min.
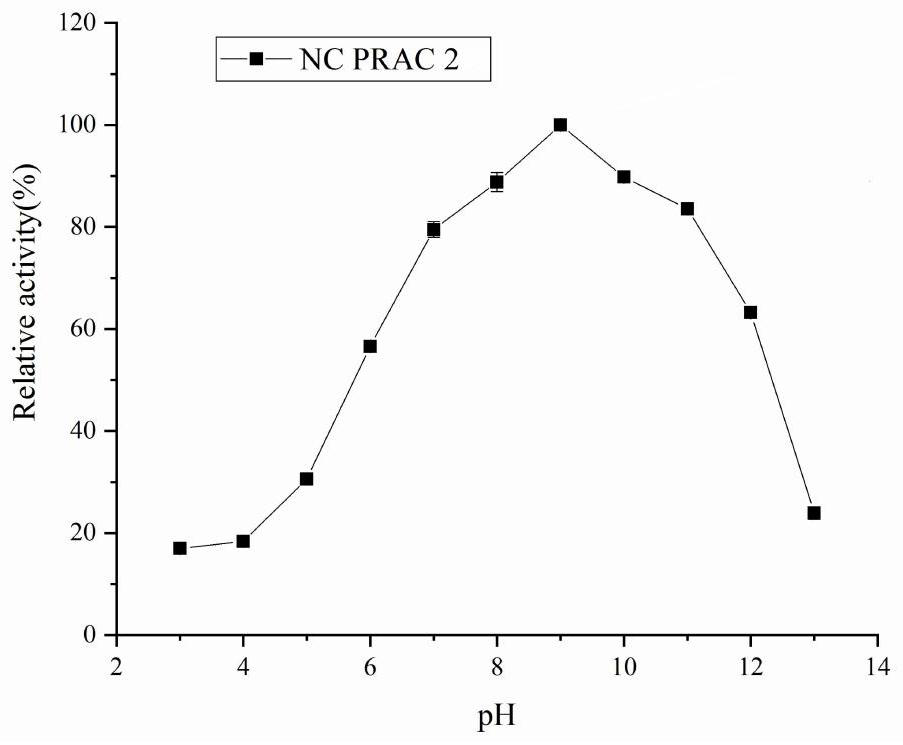
[0056] (1) Determination of optimal pH and pH stability of proline racemase NCPRAC2
[0057] Optimum pH determination of the enzyme: The proline racemase NCPRAC2 purified in Example 2 was subjected to enzymatic reaction at 37°C in a buffer of pH 3-pH 13.
[0058] Determination of pH stability of the enzyme: The purified enzyme solution was placed in a buffer solution of pH=3-13, treated at 37°C for 1 h, and then subjected to enzymatic reaction, and the untreated enzyme solution was used as a control.
[0059] The buffer solution was: 20 mmol / L Brittan-Robinson buffer solution (pH=3-13). Using L-proline a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com