Immune cell therapeutic agent for atherosclerotic plaque as well as preparation method and application thereof
A technology for immune cell therapy and atherosclerosis, which is applied in the field of cell engineering, can solve the problem of insignificant treatment effects such as dose adoption, achieve excellent gene load and transfection ability, good application prospects, and no obvious cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
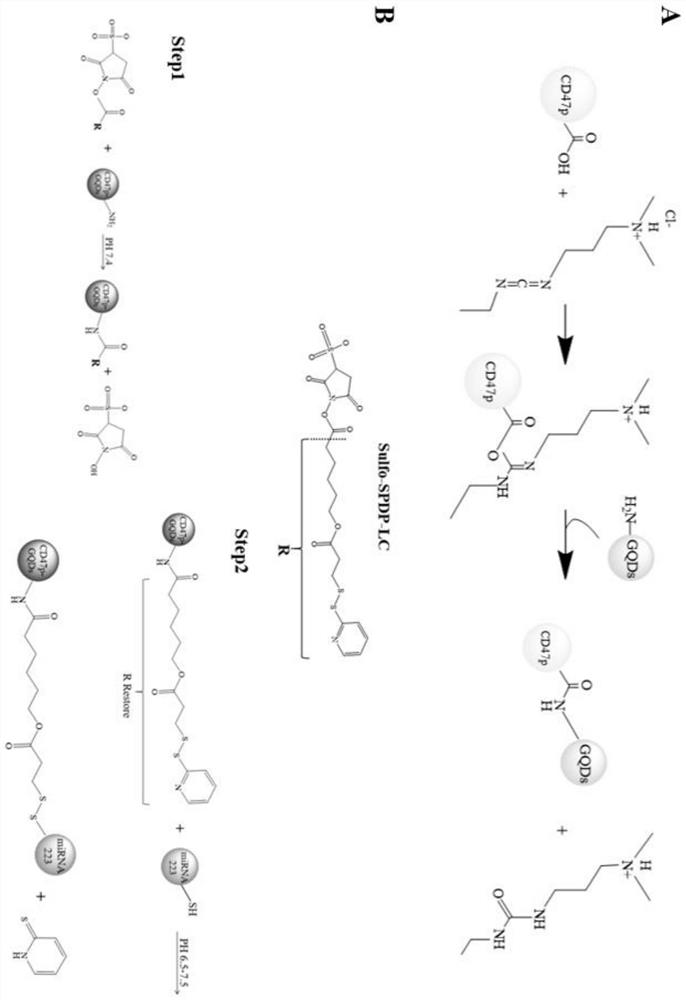
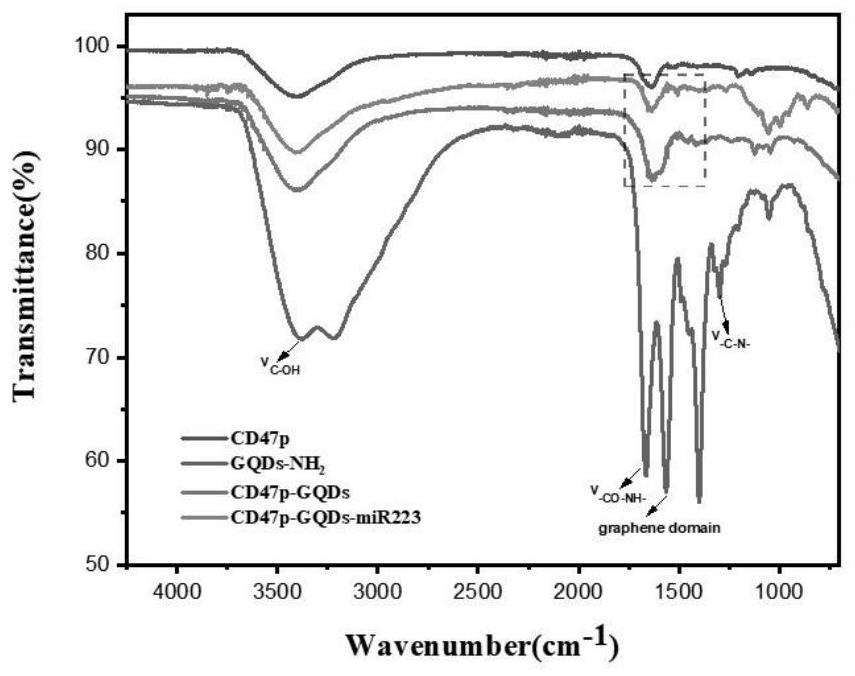
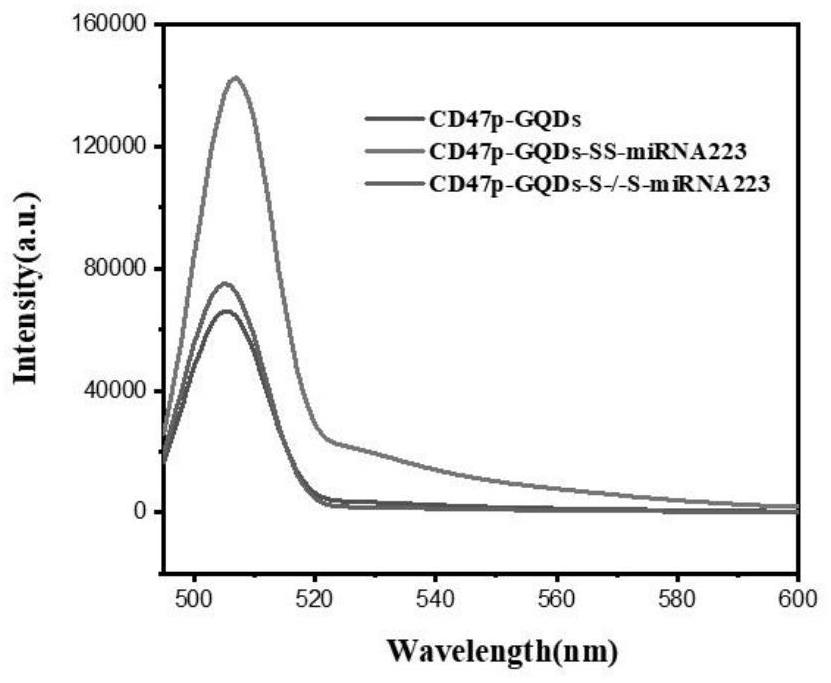
[0050] Preparation of immune cell therapeutic agent M-CD47p-GQDs-miRNA223 for atherosclerotic plaque (see appendix for the synthesis route). figure 1 )
[0051] (1) Dissolve 1 mg of CD47p powder in 1 ml of 75% ACN and 25% H 2 A 1 mg / mL CD47p solution was prepared in the mixed solution of O, and then 20 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) and 10 mg of N-hydroxysuccinimide were added in sequence. (NHS) in CD47p solution, stir magnetically until completely dissolved, adjust the pH value to be about 6.0, and react at room temperature for 30 min to obtain solution A;
[0052] (2) 1 mg / mL GQDs-NH with 5×PBS solution 2 The stock solution was diluted to 0.1mg / mL, and then 1ml of GQDs-NH was taken 2 The diluent was added to solution A, and the reaction was slightly stirred at 20-25 °C for 24 h to obtain solution B;
[0053] (3) Dialyze solution B with a dialysis bag with a molecular weight cut-off of 1kDa for 48h, and replace the dialysate every 12h to remove r...
Embodiment 2
[0061] Phagocytosis test of immune cell therapy M-CD47p-GQDs-miRNA223 for atherosclerotic plaque
[0062] (1) M-CD47p-GQDs-miRNA223, an immune cell therapy for atherosclerotic plaques, at 37°C, 5% CO2 Construct 12h under conditions;
[0063] (2) After 12h, discard the spent cell culture medium, wash it three times with PBS, and add it at a density of about 1x10 5 / ml yellow polystyrene fluorescent particles in fresh complete medium, co-cultivated with the immune cell therapeutic agent of the present invention for 6h;
[0064] (3) After 6h, discard the spent cell culture medium and wash with PBS three times. Subsequently, the nuclei were stained with DAPI with DAPI staining solution containing mounting solution for 10 min and mounted; fluorescence images were taken under a laser confocal microscope, and the results are shown in the appendix. Image 6 shown.
[0065] (4) Interpretation of the results:
[0066] attached Image 6 It can be seen that the immune cell therapeuti...
Embodiment 3
[0068] Homing ability detection of immune cell therapy M-CD47p-GQDs-miRNA223 in atherosclerotic plaques
[0069] The blank group (M+DMEM), the control group (M+MCP-1) and the experimental group (M-CD47p-GQDs-miRNA223+MCP-1) were set up respectively.
[0070] (1) Glue laying: put the transwell chamber into a 24-well plate, add 100 μL of Matrigel glue diluted with serum-free medium at a ratio of 1:8 to the upper chamber, and solidify at 37°C for 4 hours;
[0071] (2) RAW264.7 monocytes (blank group and control group) and M-CD47p-GQDs-miRNA223 cells (experimental group) were prepared to a density of 1×10 5 cells / ml single cell suspension and add 100 μL to the upper chamber of the transwell chamber, 37°C, 5% CO 2 Incubate for 6h under conditions to make cells adherent;
[0072] (3) 500 μL of monocyte chemokine MCP-1 with a concentration of 200 μg / ml was added to the lower chamber of the transwell chamber of the control and experimental groups respectively, and 500 μL of DMEM com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com