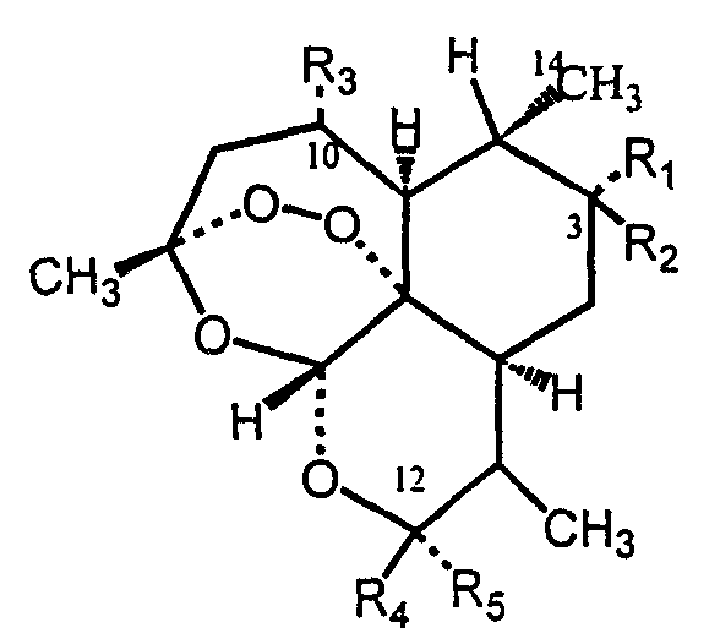
Biotransfer process for preparing artemisine compounds
A technology for artemisinin and compounds, which is applied in the field of biotransformation and preparation of artemisinin compounds, and can solve the problem of no other uses being retrieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Screening of microbial strains with transforming activity
[0048] In the present invention, 14 strains (strains) are transformed and screened, and the result shows that Streptomyces griseus (ATCC-13273) has strong transformation ability to artemisinin, dihydroartemisinin and artemether.
[0049] Liquid medium for strain screening—soybean flour glucose medium: take 20g of glucose, 5g of yeast extract, 5g of soybean flour, 5g of NaCl, K 2 HPO 4 5g, distilled water 1000mL, adjust the pH to 7.0 with 6N HCl. Pack in 150ml Erlenmeyer flasks, 25ml per bottle, sterilize at 121°C, 0.15Mpa for 20min.
[0050] When screening strains, the slant solid medium used for preserving strains is to add 1.5% agar to the above-mentioned liquid medium, heat and dissolve, then divide into 15mL test tubes with screw caps, and sterilize at 121°C and 0.15Mpa for 20min. , Obliquely cooled.
[0051] Strain preservation and activation
[0052] The strains were inoculated on the slant solid me...
Embodiment 2
[0081] Biotransformation of artemisinin compounds
[0082] Experimental Materials and Instruments
[0083] Microorganisms The microorganisms used in this experiment are Streptomyces griseus, the preservation number of which is ATCC-13273, which has been disclosed in US Patents 5,674,714, 5,213,971, and 5,169,755.
[0084] Reagents and reagents Artemisinin was prepared according to literature methods, see: Chen Yougen, Yu Boyang, etc., Extraction, separation and identification of artemisinin and its precursor compounds, Chinese herbal medicine, 2001; 32(4): 302-303, and identified by spectrum Its structure is consistent with the literature reports; dihydroartemisinin was purchased from Beijing Sixth Pharmaceutical Factory; artemether was purchased from Kunming Pharmaceutical Co., Ltd. The three compounds were respectively dissolved in acetone to make a solution with a concentration of 240 mg / mL, shaken well, and used as conversion substrates for later use.
[0085] Medium 20%...
Embodiment 3
[0120] Selection of Carbon Source, Nitrogen Source and Metal Ion in Artemisinin Microbial Transformation Medium
[0121] The main product of artemisinin transformed by microorganism Streptomyces griseus was screened, especially the transformation conditions of 3α-hydroxyartemisinin.
[0122] Medium:
[0123] Carbon source selection medium: 20% potato decoction 1000ml, carbon source (glucose or soluble starch, sucrose, maltose, citric acid, galactose, dextrin, corn flour, glycerin) 20g, yeast extract 5g, soybean powder 5g , KH 2 PO 4 3.0g, MgSO 4 ·7H 2 O 1.5g, VB 1 Trace amount (pH5.0~7.5).
[0124] Nitrogen source selection medium: 20% potato decoction 1000ml, glucose 20g, nitrogen source (peptone or beef extract, yeast extract, corn flour, soybean flour 10g, or urea, ammonium carbonate, sodium nitrate, L-lysine , DL-α-alanine is equivalent to sodium nitrate 2g / L), KH 2 PO 4 3.0g, MgSO 4 ·7H 2 O 1.5g, VB 1 Trace amount (pH5.0~7.5).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com