Human melanin tumour monoclonal antibody, and its preparing method and use
A monoclonal antibody, melanoma technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc. and the problem of unsatisfactory neutralization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Screening of Human Melanoma Antigens
[0047] 1. Construction of cDNA library of melanoma cell line A2058
[0048] Total RNA was extracted from human melanoma A2058 cells (ATCC, NO.CRL11147) with Trizol reagent, mRNA was separated by Oligo (T) fiber column chromatography, and the extracted mRNA was reverse-transcribed into cDNA with random primers, filled with BstXI The linkers at the restriction sites were connected, and cloned into the mammalian transient expression vector pCDM8 (Invitrogen) after digestion with BstXI, and transformed into Escherichia coli MC1061 / P3 (purchased from Invitrogen Company) to form a cDNA library.
[0049] 2. Library expression and screening.
[0050] Spread COS-7 cells (Invitrogen) into 20 10cm petri dishes, use the cDNA library constructed above to transfect according to the lipofectin method, and after 12 hours, the cells were digested with trypsin and respread in new petri dishes, transfected After staining for 72 ...
Embodiment 2
[0058] Screening the Variable Region Gene of Human Anti-Melanoma Monoclonal Antibody hSM5-1 from Human Antibody Library
[0059] According to Marks et al. J. Mol. Biol., 222, 581-597; Hoogenboom and Winte, J. Mol. Biol., 227, 381-388; Haidaris CG et al., J Immunol Methods. 2001 Nov 1; 257 (1- 2): 185-202; Griffiths, A.D. et al. EMBO J., 13, 3245-3260 (1994); Nissim, A. et al. The method described in EMBO J., 13, 692-698 (1994) to construct a human antibody library .
[0060] Add 1 ml of the revived antibody library strain to 14 ml of fresh LB medium, and culture in a 50 ml Erlenmeyer flask at 37°C for 16 hours.
[0061] Centrifuge at a high speed of 12000rpm for 10 minutes, transfer the supernatant to a sterile 50ml centrifuge tube, and save it for future use. Its titer should be 2×10 11 above. A 25 ml cell culture flask was coated with the antigen A220 purified in Example 1. Add no less than 3×10 10 Phage particles were incubated at 37°C for 1 hour. Then, the liqu...
Embodiment 3
[0068] Expression of Human Anti-Melanoma Monoclonal Antibody
[0069] 1. Construction of expression vector
[0070] The strains of the above positive clones were amplified in 100 ml of LB medium, and the plasmid DNA was purified with a plasmid DNA extraction and purification kit from Promega Company.
[0071] After digesting the above plasmid DNA with XbaI and NheI, separate the digested fragments on 1.5% agarose gel electrophoresis, take about 350 bp band for gel recovery, and the obtained fragment is the light chain variable region coding sequence.
[0072] The above plasmid DNA was digested with HindIII and Bsi WI, and the digested fragments were separated on 1.5% agarose gel electrophoresis, and a band of about 320 bp was taken for gel recovery, and the obtained fragment was the heavy chain variable region coding sequence.
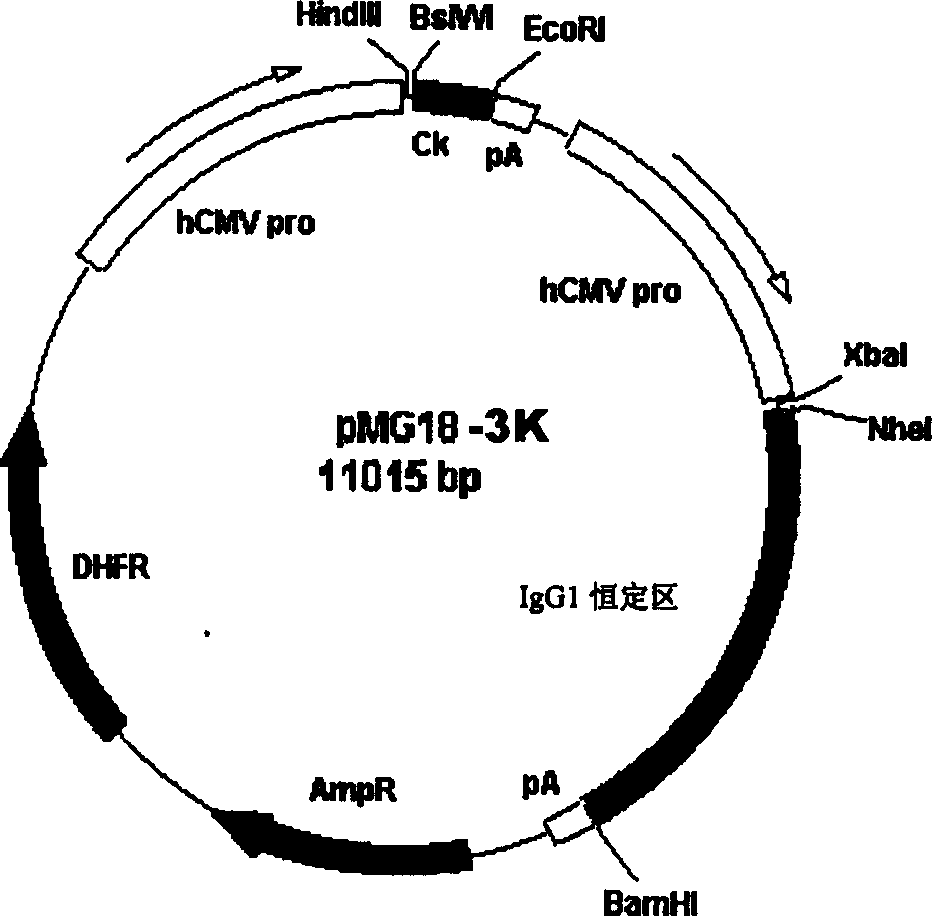
[0073] Then first insert the above-mentioned light chain variable region coding sequence into the expression vector pMG18-3K (see DEVEL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com