Method of contact printing on gold coated films
A film and polymer film technology, applied in the field of micro-contact printing, can solve the problems of insufficient bonding and hindering self-integration of monolayers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
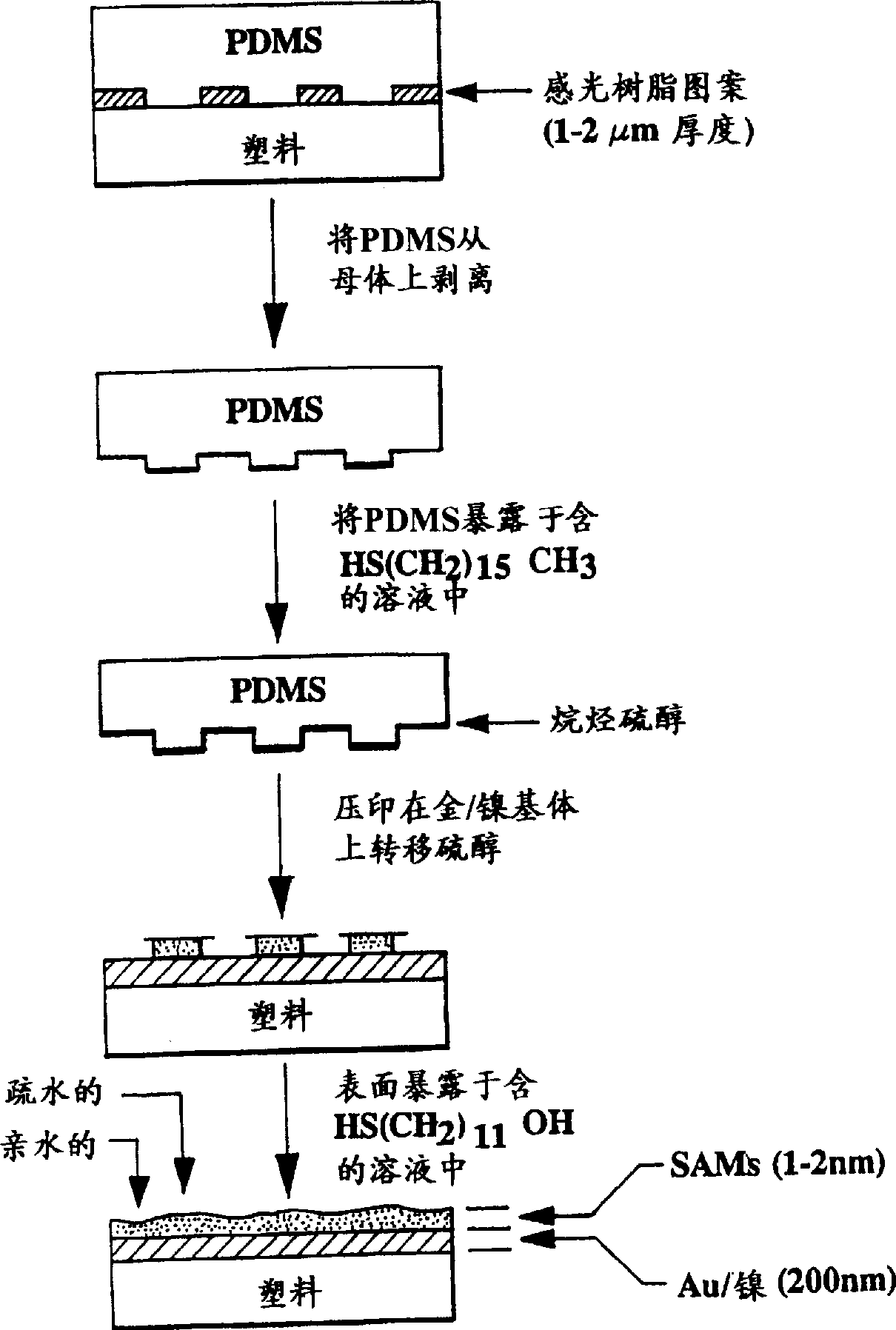
[0042] Gold-plated MYLAR(R) (polyethylene terephthalate) was printed with a pattern of 16-mercaptohexadecanic acid and cetylmercaptan.
[0043] Pattern printing of gold-plated MYLAR® (polyethylene terephthalate) with a pattern of 16-mercaptohexadecanic acid and cetyl mercaptan, such as figure 1 shown, and described below.
[0044] MYLAR(R) film modified with a plasma deposited gold topcoat was obtained from Courtaulds Performance Films (21034 Osborne Street, Canoga Park, CA 91304). figure 2 An atomic force microscope image of this MYLAR thin film is shown. A polymer film with a thickness of 2-7 mils and a gold overcoat that yields a surface resistance of 65 ohms per square centimeter and a visible light transmission of 20%-65% is used.
[0045] Hydrophilic carboxy-terminated alkanethiols were imprinted onto gold-coated films using 16-mercaptohexadecanoic acid by the following method. Light exposure of 10 micron diameter rings on a silicon wafer and the resulting photoresis...
Embodiment 2
[0048] Aluminum-coated MYLAR(R) was printed with 16-carboxyhexadecanoic acid and cetyl carboxylate.
[0049] With 1,16-hexadecane dihydroxamic acid and 1-hexadecane hydroxamic acid respectively replace the hydrophilic and hydrophobic mercaptan in embodiment 1, with 35% visible light transmittance to 100 standard Aluminum coated MYLAR(R) The method of Example 1 was followed. Diffraction of visible light. Reflection and transmission diffraction patterns can be observed when using 5mM, 670nM laser irradiation. Rainbow diffractive colors can be observed with transmitted white light. Example 3
Embodiment 3
[0050] The gold-coated MYLAR(R) was compared to a gold-coated silicon wafer.
[0051] Thin films of gold (100 angstroms to 1 micron) were deposited by electron beam evaporation on silicon wafers that had been pre-coated with titanium (5-50 angstroms) to promote adhesion between silicon and gold. Imprinting was performed as in Example 1 on gold-coated films and gold-coated silicon wafers.
[0052] Measurement of contact angle
[0053]The contact angle was measured with a Rame-Hart 100 goniometer at room temperature and ambient humidity. Water for contact angles was deionized and distilled in glass and taffeta apparatus. The advancing and receding contact angles of at least three drops of each liquid were measured on both sides of each slide, and the data in the figure represent the average of these measurements. Contact angles were measured using the following method: A drop with a volume of 1-2 microliters was formed on the end of a pipette (Micro-Electrapettesyringe, Matri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com