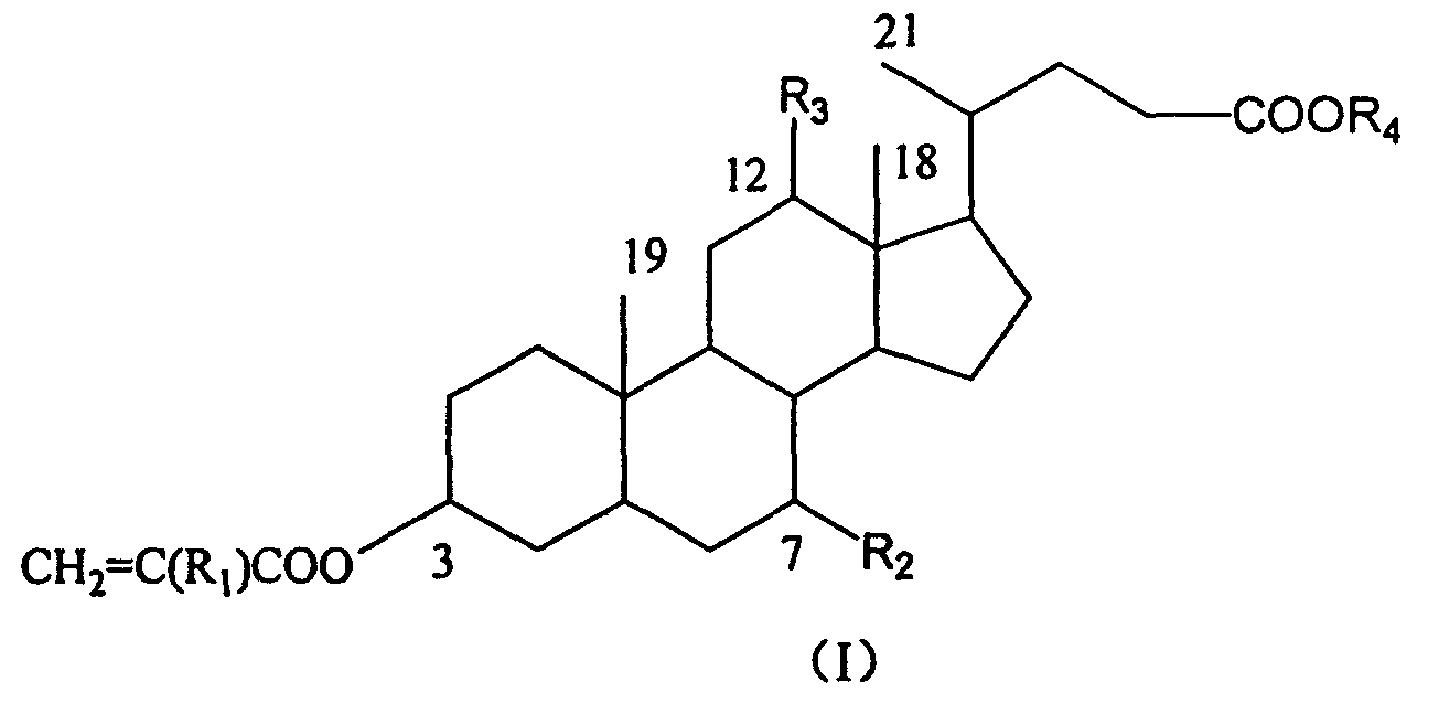
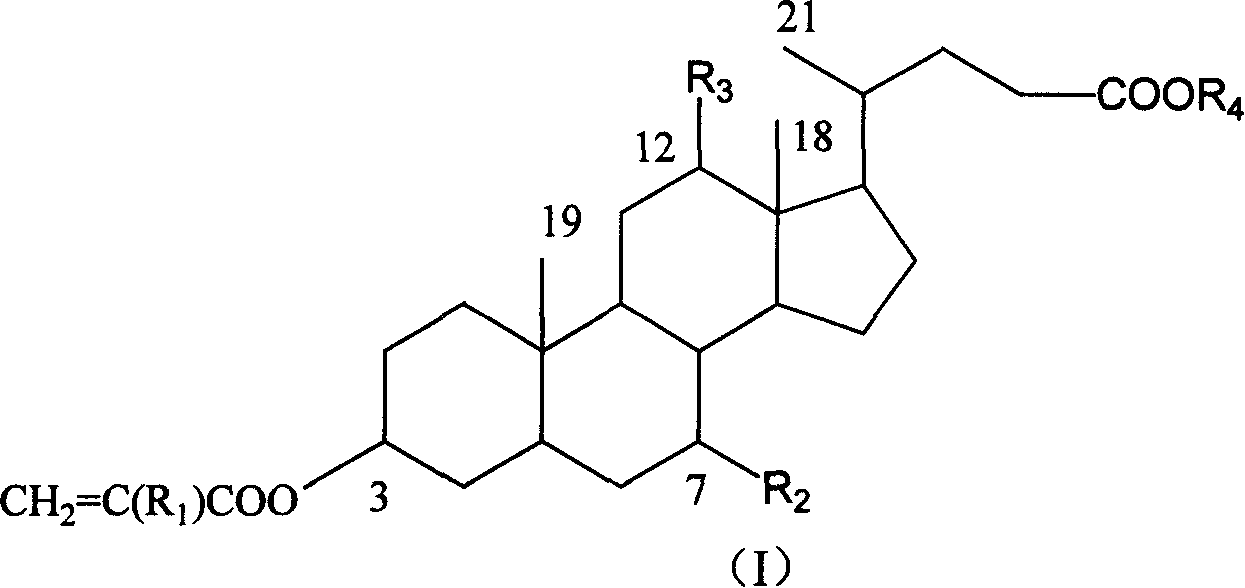
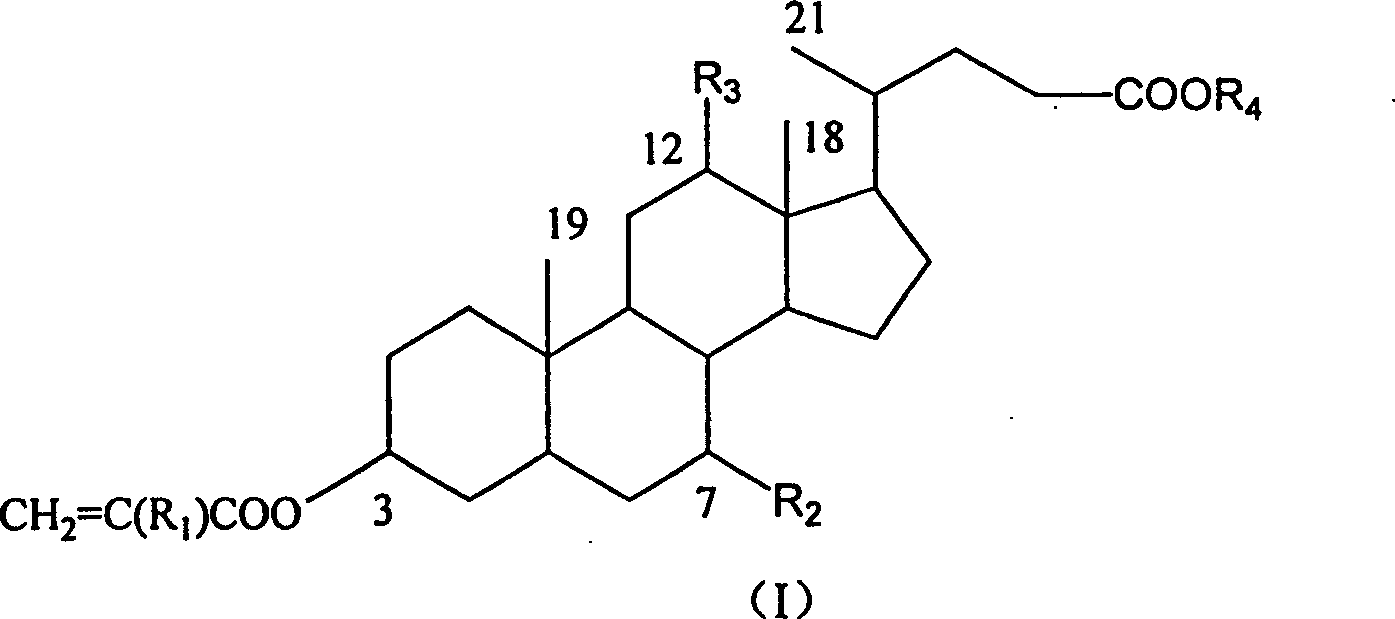
Crosslinking monomer containing bile acid and preparation process and use thereof
A technology for cross-linking monomers and bile acids, which is applied in the directions of organic chemistry methods, chemical instruments and methods, prostheses, etc., to achieve the effects of high yield, small polymerization shrinkage rate and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 25g of cholic acid methyl cholic acid in 125mL of methanol, add 0.5ml of concentrated hydrochloric acid, reflux for 20min., cool to 0°C, crystals are precipitated, and the product is obtained by suction filtration. Yield 93%. (Reference Steroids 61 (1996) 664).
Embodiment 2
[0023] Add 10.0 g of cholic acid into a 250 mL three-necked bottle of cholic acid (2′-hydroxyethyl), stir 80 mL of ethylene glycol and raise the temperature to 58° C., then add 0.5 mL of concentrated hydrochloric acid dropwise. Reaction 10h. Add water to precipitate out. Suction filtration, washing with sodium carbonate aqueous solution, water washing pH to 7.0, vacuum drying, to obtain 10.1 g, yield 91%. 1 H-NMR (400MHz, CDCl 3 ) (part): δ / ppm=0.69(s, 3H, 18-H 3 ), 0.89(s, 3H, 19-H 3 ), 1.00 (d, 3H, 21-H 3 ), 3.43(m, 1H, 3α-H), 3.82(m, 3H, 7α-H, COOCH 2 CH 2 OH), 3.97(s, 12α-H), 4.21(t, 2H, COOCH 2 CH 2 OH). Elemental Analysis C 26 H 44 O 6 (FW: 452.63), Calculated: C% 68.99, H% 9.80; Found: C% 68.84, H% 9.87
Embodiment 3
[0024] Example 3 Synthesis of 3α, 12α-dimethylacryloyl cholate
[0025] Dissolve 1.00 g of methyl cholate in a sufficient amount of THF in a 100 mL three-neck flask. While stirring in an ice-water bath, 1.48 g of methacryloyl chloride and 2.39 g of triethylamine in THF were added dropwise. The ice-water bath was removed, and the reaction was carried out at 20° C. for 12 hours. TLC detected that the reaction was complete. Triethylamine hydrochloride was filtered off and THF was evaporated. It was purified by silica gel column chromatography, and the eluent was petroleum ether / ethyl acetate (20 / 7) to obtain 0.61 g of the product with a yield of 46%. 1 H-NMR (400MHz, CDCl 3 ) (part): δ / ppm=0.77(s, 3H, 18-H 3 ), 0.83(d, 3H, 21-H 3 ), 0.91(s, 3H, 19-H 3 ), 1.91(s, 3H, C(CH 3 )=CH 2 ), 1.99(s, 3H, C(CH 3 )=CH 2 ,), 3.65(s, 3H, COOCH 3 ), 3.89(s, 7α-H), 4.60(m, 1H, 3α-H), 5.17(s, 1H, 12α-H), 5.50(s, 1HC(CH 3 )=CH), 5.57(s, 1H C(CH 3 )=CH), 6.04(s, 1H, C(CH 3 )=CH), 6.14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com