Parathyroid hormone analog and its preparing process by recombination
A parathyroid hormone, analog technology, applied in the direction of parathyroid hormone, hormone peptides, drug combinations, etc., can solve the problems of loss of activity, long half-life, no biological activity, etc. Yield improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] Example 1: Parathyroid hormone analog PTH (1-34) (Gly 1 Gln 26 ) gene acquisition
[0014] PTH(1-34)(Gly1G l no 26 ) (hereinafter referred to as PTHa) amino acid sequence is as follows:
[0015] Seq1: (amino acid sequence of PTHa protein):
[0016] Gly-Val-Ser-Glu-Ile-Gln-Leu-Met-His-Asn-Leu-Gly-Lys-His-Leu-Asn-Ser-Met-Glu-Arg-Val-Glu-Trp-Leu-Arg- Gln-Lys-Leu-Gln-Asp-Val-His-Asn-Phe.
[0017] When synthesizing the gene sequence, according to the degeneracy principle of amino acid codons and the principle of E. coli preferred codons, the designed template single strand is as follows (102bp):
[0018] Seq2: (PTHa gene single-stranded template sequence):
[0019] 5′GGT GTT TCC GAA ATC CAG TTG ATG CAT AAC TTG GGT AAA CAT TTG AAC
[0020] TCC ATG GAG AGA GTT GAA TGG TTG AGA CAA AAG TTG CAG GAT GTT CAC
[0021] AAT TTT 3'.
[0022] Primers can be designed according to different fusion expression needs:
[0023] 1. Design of PCR primers for fusion expression o...
example 2
[0039] Example 2: Construction and expression of recombinant PTHa fusion expression vector
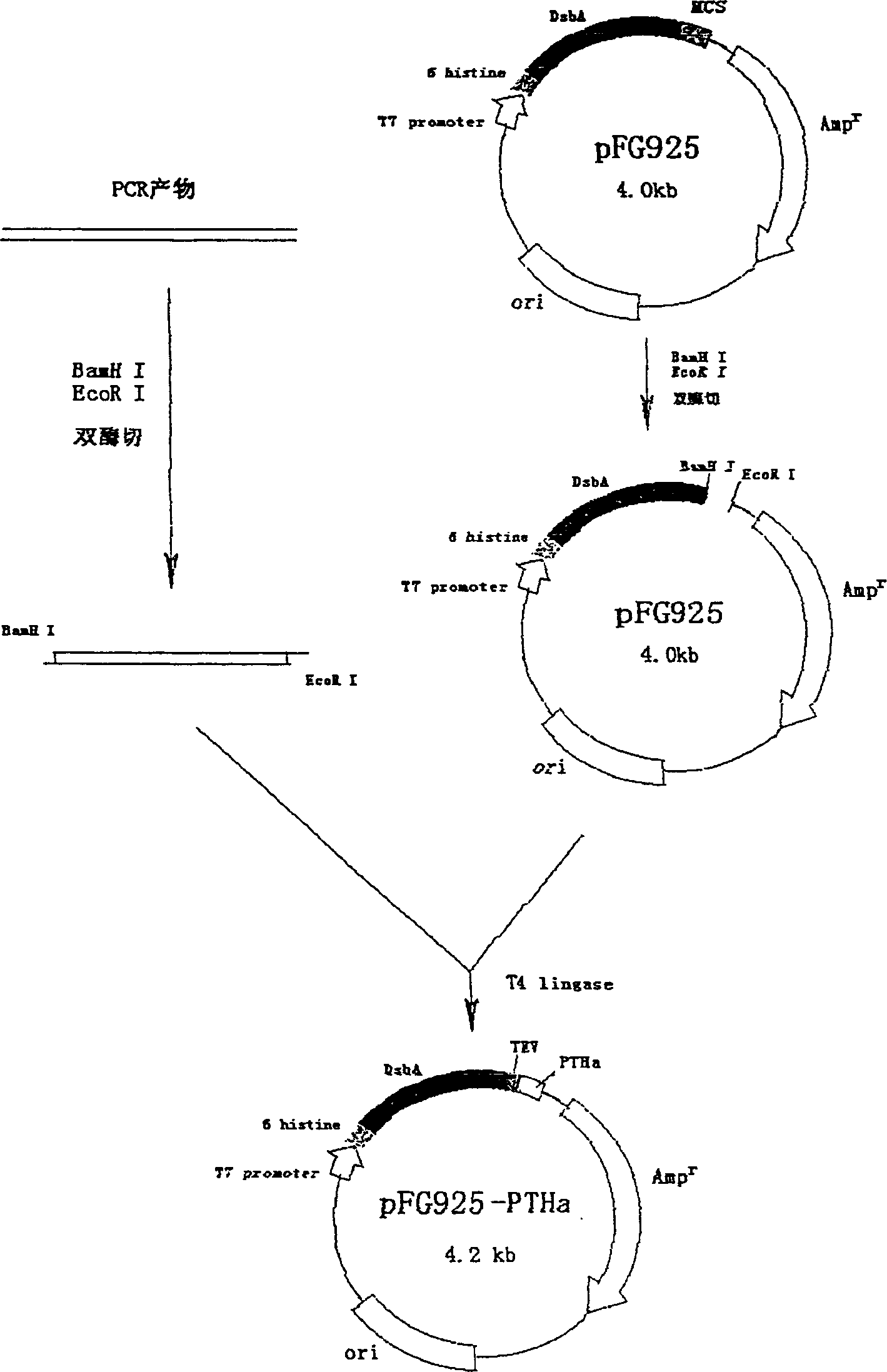
[0040] 1. Construction of DsbA fusion expression PTHa vector for TEV digestion and acquisition of engineering bacteria
[0041] The PCR product amplified with primers Seq3 and Sep4 was digested with BamH I and EcoR I, and the pFG925 plasmid after double digestion with BamH I and EcoR I was ligated with T4 ligase at 14°C, and the recombinant plasmid was named pFG925 -PTHa( figure 1 ). Then use CaCl 2 coli BL21(DE3)pLysS, spread on SOB plates containing 50 μg / mL ampicillin and 35 μg / mL chloramphenicol, select positive colonies and culture them in SOB until OD 600 When it was 0.6-0.8, add IPTG 1.0mM to induce 3-4 hours and centrifuge to collect the bacteria. After breaking the bacteria with 8M urea, a protein band of about 52KD appeared in 10% SDS-PAGE electrophoresis, and the expression level was 45-50%. The monoclonal antibody (DSL product, USA) of the natural type PTH (1-34) was dete...
example 3
[0044] Example three: Purification of recombinant PTHa
[0045] 1. Purification of DsbA fusion expression PTHa
[0046] The expression of recombinant PTHa protein is accomplished by using the BL-PTHa engineering bacteria constructed by our company. 20LM9CA bacteria were collected after fermentation, sonicated and dissolved with 6M urea, centrifuged and diluted 4 times with 50mM Tris-HCl (pH8.0). The sample passes through Ni + The fusion protein was collected by gradient elution with 50mM-200mM Imidazone after Chelating Sepharose Fast Flow, and the purity reached 90%. It was digested with TEV enzyme (GIBCO company product) at 30°C for 1 hour, and then passed through ChelatingSepharose Fast Flow DsbA protein was removed, and the excised recombinant PTHa was treated with C 8 Reverse phase chromatography obtained recombinant PTHa with a purity greater than 95%.
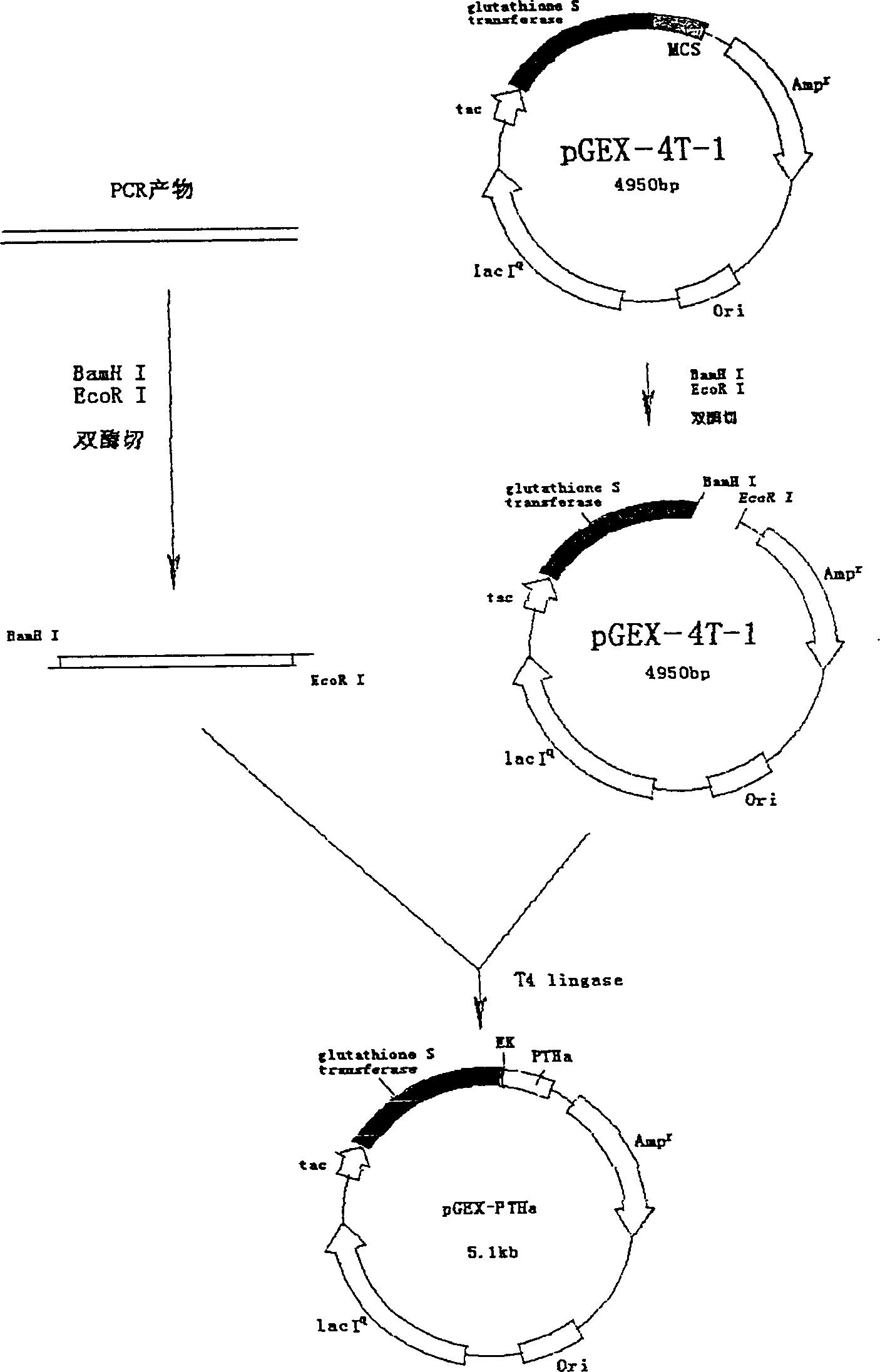
[0047] 2. Purification of GST fusion expression PTHa
[0048] The expression of recombinant PTHa protein was accom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com