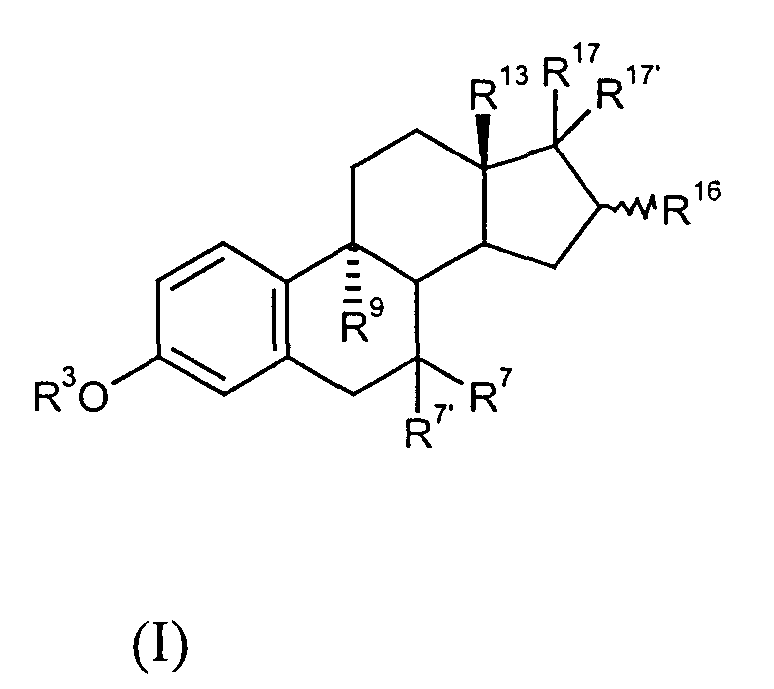
9-alpha-substituted estratrienes as selectively active estrogens
A representative, alkenyl-based technology, applied in the field of 9α-substituted estratriene as a selective estrogen, can solve the problem of not showing estrogen receptor selectivity in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] 9α-Vinylestradiol-1,3,5(10)-triene-3,16α-diol
[0175] step 1
[0176] 9α-cyano-3-methoxy-estra-1,3,5(10)-trien-16α-yl-acetate
[0177] A solution of 2.21g (9.73mmol) of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in 80ml of dichloromethane was added dropwise to 2.13g (6.49mmol) under stirring. 3-methoxy-estra-1,3,5(10)-trien-16α-yl-acetate, 2.07ml (16.54mmol) of trimethylsilyl cyanide and 0.14g of perchlorate Lithium oxide was suspended in 100 ml of dichloromethane. The reaction mixture is green. After 1 hour at room temperature, the mixture was admixed with sodium bicarbonate solution. The separated organic phase was washed with water and evaporated in vacuo. The product mixture is chromatographed on silica gel (cyclohexane / ethyl acetate, 6 / 1). This gave 0.44 g (21%) of 9α-cyano-3-methoxy-estra-1,3,5(10)-trien-16α-yl-acetate.
[0178] step 2
[0179] 9α-cyano-3-hydroxy-estra-1,3,5(10)-trien-16α-yl-acetate
[0180] Under argon atmosphere, 7.51 g (50.1 mmol) of s...
Embodiment 2
[0193] 9α-vinyl-18a-homo-estra-1,3,5(10)-triene-3,16α-diol
[0194] step 1
[0195] 3,16α-bis[(perhydropyran-2-yl)oxy]-18a-homo-estra 1,3,5(10)-triene-9-carbonitrile
[0196] 1.03g (2.26mmol) of 3,16α-bis[(perhydropyran-2-yl)oxy]-18a-homo-estra-1,3,5(10)-triene, 48.2 mg (0.45 mmol) of lithium perchlorate and 0.71 ml (5.66 mmol) of trimethylsilyl cyanide were introduced into 10 ml of dichloromethane (molecular sieves) and cooled to about -70° C. while stirring. A solution of 0.77 g (3.39 mmol) of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in 65 ml of dichloromethane was added dropwise over a period of 1 hour. After about 1 hour (warming to room temperature), the reaction mixture was mixed with sodium bicarbonate solution, and the reaction product was extracted with dichloromethane. The crude product obtained by concentrating the organic phase by evaporation is purified by chromatography. After chromatography on silica gel (cyclohexane / ethyl acetate, 4 / 1), 0.74 g (68% of theo...
Embodiment 3
[0208] 9α-(2′,2′difluorovinyl)-estra-1,3,5(10)-triene-3,16α-diol
[0209] step 1
[0210] 3,16α-bis[(perhydropyran-2-yl)oxy]-estra-1,3,5(10)-triene-9-carbonitrile
[0211] Similar to Step 1 of Example 1, 3,16α-bis[(perhydropyran-2-yl)oxy]-estra-1,3,5(10)-triene was reacted to produce 3,16α- Bis[(perhydropyran-2-yl)oxy]-estra-1,3,5(10)-triene-9-carbonitrile.
[0212] Yield: 58% of theory
[0213] step 2
[0214] 3,16α-Dihydroxy-estra-1,3,5(10)-triene-9-carbaldehyde
[0215] Similar to step 2 of Example 1, 3,16α-bis[(perhydropyran-2-yl)oxy]-estra-1,3,5(10)-triene-9-carbonitrile was reacted, 3,16α-Dihydroxy-estra-1,3,5(10)-triene-9-carbaldehyde is produced.
[0216] Yield: 83% of theoretical value
[0217] step 3
[0218] 9α-(2,2-difluorovinyl)-estra-1,3,5(10)-triene-3,16α-diol
[0219] 1.5 ml of dimethoxyethane (molecular sieves), 0.3 ml of pentane and 0.13 ml (0.77 mmol) of diethyl(difluoromethyl)-phosphate were introduced into an inert reaction flask and cooled to ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com