Photothermally chemical process for synthesizing iodoperfluoroparaffin
An iodoperfluoroalkane, photothermal chemistry technology, applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation, etc., can solve the problems of low photoreaction quantum efficiency, short reactor life, low reaction speed, etc. To achieve the effect of increased reaction speed, easy separation and extended service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
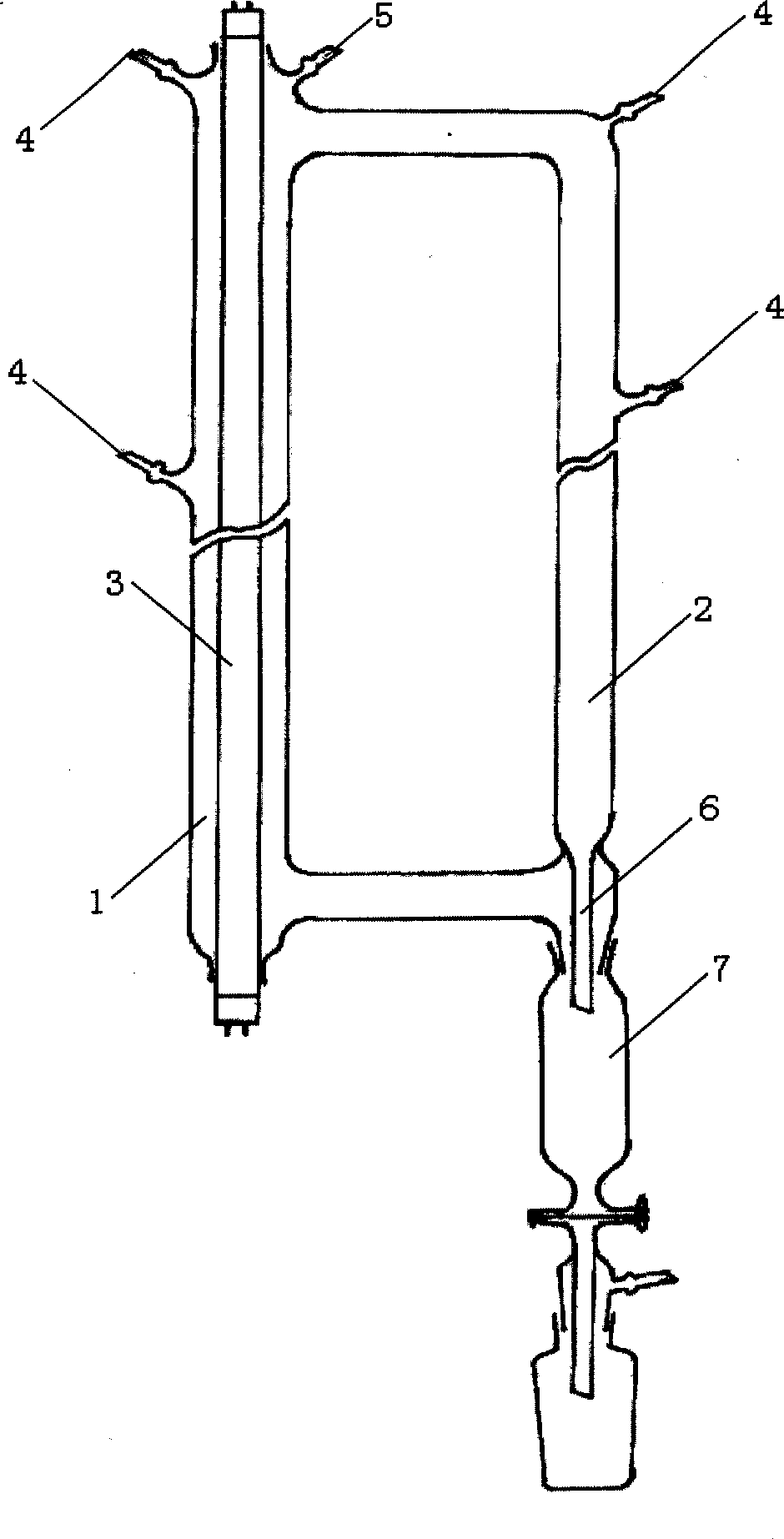
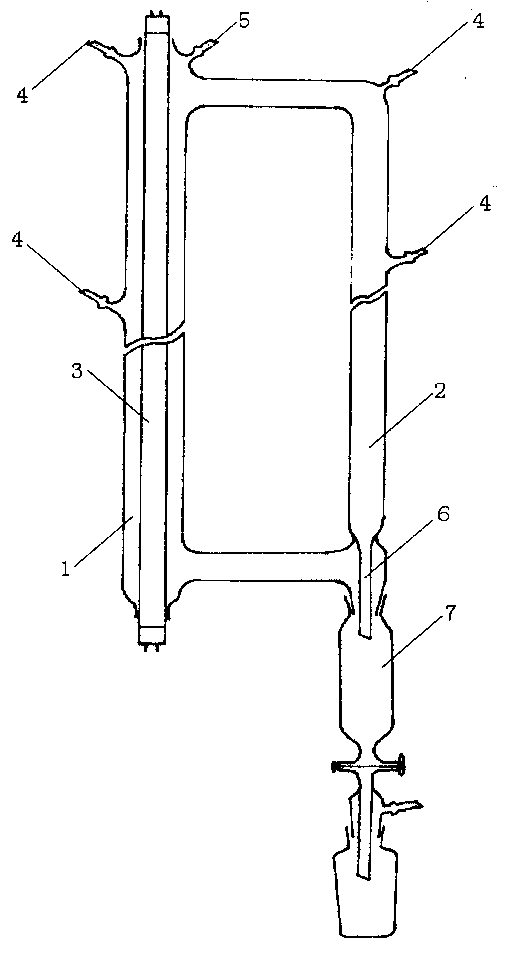
[0023] Use as attached figure 1 The photothermochemical reactor shown. Depend on figure 1 It can be seen that the reactor consists of two interconnected cylindrical tubes. The reaction tube 1 is 900 mm long and its inner diameter is 60 mm. A 40W ozone-free low-pressure mercury lamp 3 is sealed in the middle, and a conductive tape is wrapped around the outside. The inner diameter of the heat circulation tube 2 is 40 mm. , the outside is also wrapped with conductive tape, and the terminal posts at both ends of the lamp tube are led out by wires and connected to the electronic ballast. On the upper end of the reaction tube 1, there are feeding port 4, vacuum system connection port 5, reaction tube 1 and thermal cycle tube 2 are equipped with a material inlet 4 in the middle, and a material outlet 6 and a receiver 7 are arranged at the lower end of the heat circulation pipe 2 .
[0024] Add 20 grams of C to receiver 7 4 f 9 I and C 6 f 13 I mixture (wherein C 4 17.5%, C 6 ...
Embodiment 2
[0026] The reactor and operation steps are the same as in Example 1, the reaction temperature is 200°C (reaction tube 1), the total pressure of the reaction system is controlled at 80Kpa, and C 2 f 4 with C 2 f 5 The total molar ratio of I was 2.92, and the reaction was finished after 13.5 hours, and the product taken out was analyzed by gas chromatography. After analysis, the C contained in the product 2 f 5 I.C 4 f 9 I.C 5 f 11 I.C 6 f 13 I.C 7 f 15 I.C 8 f 17 I.C 9 f 19 I.C 10 f 21 I.C 12 f 25 The weight percentage composition of I is: 0.91%, 6.17%, 0.49%, 41.79%, 0.82%, 35.62%, 0.41%, 10.58%, 2.30%. Calculated from the above data to get C 2 f 5 The weight conversion rate of I is 97.2%, C 4 f 9 I.C 6 f 13 I.C 8 f 17 I.C 10 f 21 I versus C 2 f 5 The weight yields in terms of conversion of I were 6.5%, 68.6%, 110.6%, 32.9%, 7.2%, respectively. The quantum efficiency of this reaction is 0.23. In addition there are small amounts of perfluoroalk...
Embodiment 3
[0028] The reactor and operation steps are the same as in Example 1, the reaction temperature is 250°C (reaction tube 1), the total pressure of the reaction system is controlled at 100Kpa, and C 2 f 4 with C 2 f 5 The total molar ratio of I was 2.94, and the reaction was terminated after 17 hours, and the product taken out was analyzed by gas chromatography. After analysis, the C contained in the product 2 f 5 I.C 4 f 9 I.C 5 f 11 I.C 6 f 13 I.C 7 f 15 I.C 8 f 17 I.C 9 f 19 I.C 10 f 21 I.C 12 f 25 The weight percentage composition of I is: 1.70%, 5.13%, 0.35%, 22.87%, 0.55%, 38.58%, 0.45%, 21.60%, 7.77%. Calculated from the above data to get C 2 f 5 The weight conversion rate of I is 96.2%, C 4 f 9 I.C 6 f 13 I.C 8 f 17 I.C 10 f 21 I versus C 2 f 5 The weight yields in terms of conversion of I were 9.5%, 41.7%, 88.9%, 49.8%, 17.9%, respectively. The quantum efficiency of this reaction is 0.99. In addition there are small amounts of perfluoroal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com