Keratinocyte growth factor-2
A host cell, encoding technology, applied in the field of use, inhibiting the effect of this polypeptide, new mutant form, keratinocyte growth factor, and purifying KGF-2 polypeptide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0164] Epitope-bearing peptides and polypeptides of the invention can be produced by any conventional method for preparing peptides or polypeptides, including recombinant methods using nucleic acid molecules of the invention. For example, short epitope-bearing amino acid sequences can be fused to larger polypeptides that can be used as vectors during recombinant production and purification, and to generate anti-peptide antibodies during immunization. Epitope-bearing peptides can also be synthesized using known chemical synthesis methods. For example, Houghten described a simple method for the synthesis of large numbers of peptides, as 10-20 mg of 248 different peptides representing single amino acid variants of a segment of the HA1 polypeptide were prepared and characterized (by ELISA-type binding studies) within 4 weeks. 13-residue peptides, Houghten, R.A (1985), A general method for rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody int...
example
[0578] According to the present invention, any method used in the art for gene therapy can be used. The instance methods are described below.
[0579] For an overview review of gene therapy, see Goldspiel et al., Clinical Pharmacy 12:488-505 (1993); Wu and Wu, Biotherapy 3:87-95 (1991); Tolstoshev, Pharmacology and Toxicology Ann. Rev. Pharmacol. Toxicol. 32: 573-596 (1993); Mulligan, Science 260: 926-932 (1993); and Morgan and Anderson, Ann. Rev. Biochem. ) 62: 191-217 (1993); May, Trends in Biotechnology (TIBTECH 11) (5): 155-215 (1993). Applicable recombinant DNA technology methods generally known in the art are described in Ausubel et al. (eds.), Current Protocols in Molecular Biology, John Wiley & Sons, New York (1993); and Kriegler, Gene Transfer and Expression , Gene Transfer and Expression, A Laboratory Manual, Stockton Press, New York (1990).
[0580] In a preferred aspect, the compound includes a nucleic acid sequence encoding an antibody, and the nucleic acid seq...
Embodiment 1
[0757] Bacterial expression and purification of embodiment 1KGF-2
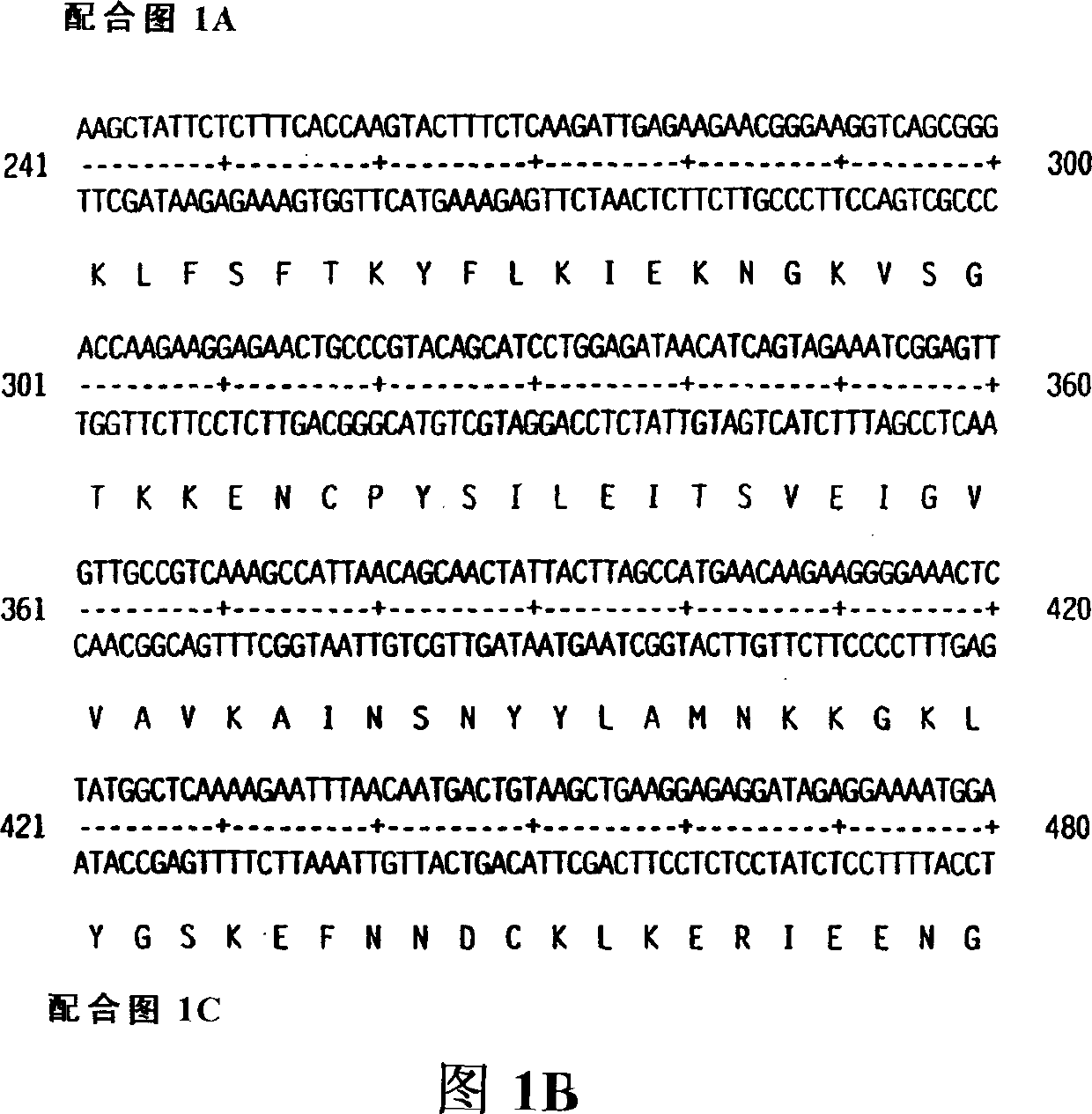
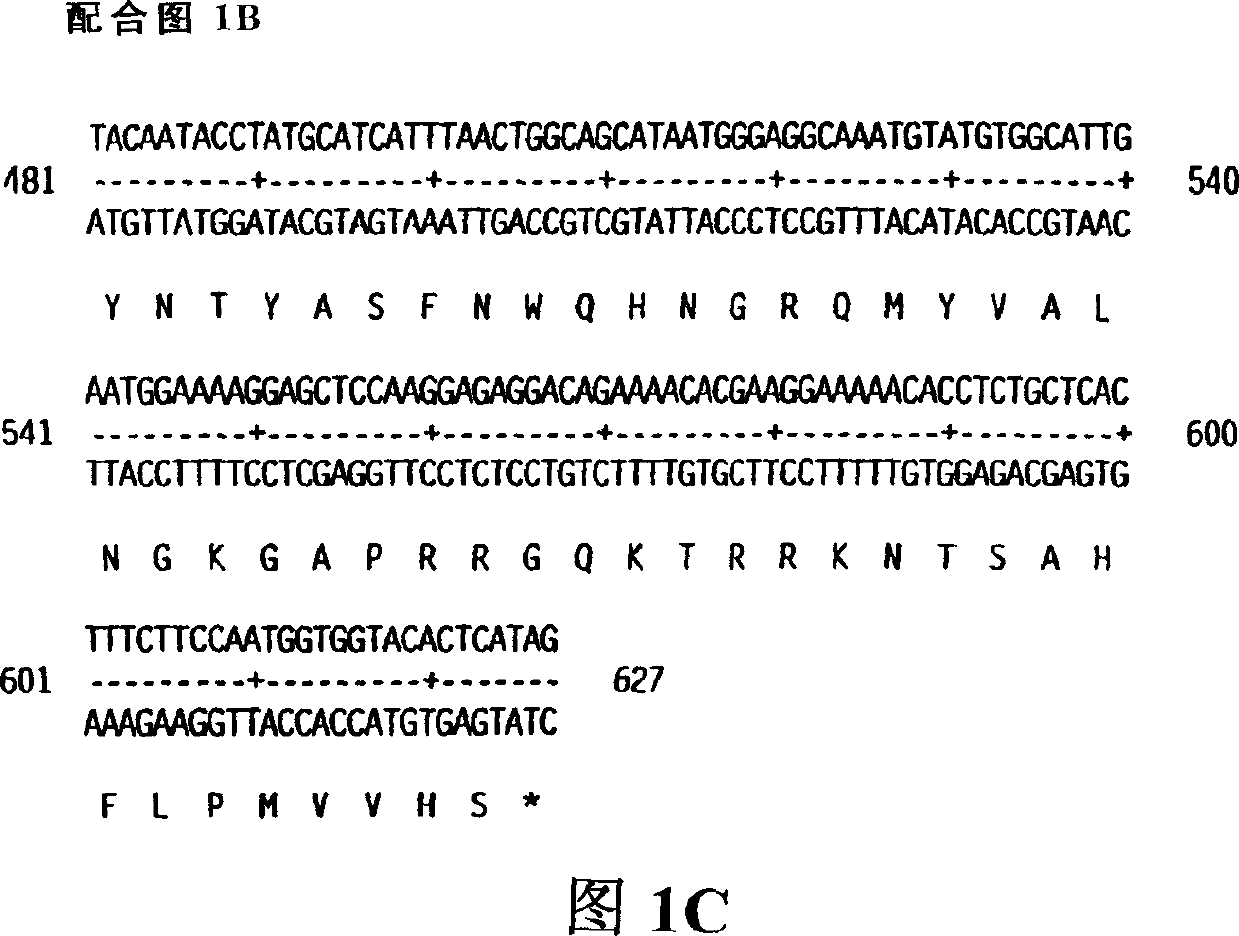
[0758] The DNA sequence ATCC #75977 encoding KGF-2 was first amplified with PCR oligonucleotide primers corresponding to the 5' and 3' sequences of the processed KGF-2 cDNA (including the signal peptide sequence). The 5' oligonucleotide primer contained the sequence 5'CCCCACATGTGGAAATGGATACTGACACATTGTGCC3' (SEQ ID NO. 3) containing an Afl III restriction enzyme site including and followed by KGF-2 starting from the putative start codon 30 nucleotides of the coding sequence. The 3' sequence 5'CCCAAGCTTCCACAAACGTTGCCTTCCTCTATGAG3' (SEQ ID NO. 4) contains the complement of the Hind III site followed by 26 nucleotides of KGF-2. These restriction enzyme sites are compatible with the restriction enzyme sites on the bacterial expression vector pQE-60 (Qiagen, Inc. Chatsworth, CA). pQE-60 encodes antibiotic resistance (AMpr), a bacterial origin of replication (ori), IPTG-regulated promoter oper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com