Method for synthesizing crystallites and block crystals of nitride by multistep reaction in-situ under hydrothermal condition
A nitride and crystal technology, applied in the direction of nitrogen-metal/silicon/boron binary compounds, etc., can solve the problems of many reducing agents, difficult reactivity, difficult reaction process, etc., and achieve strong controllability and wide applicability , the effect of eliminating pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1. prepare BN nanopowder
[0049] Using hydrazine hydrate as a reducing agent, boric acid and sodium azide react to synthesize boron nitride under hydrothermal conditions. The chemical reaction in water is as follows: (a) (b) (c) (d) (e) (in the reaction formula, with " * "indicates highly reactive atoms)
[0050] The specific operation process is as follows:
[0051] First weigh 3.1g of boric acid and add it to a conical flask with a capacity of 250ml, then add 100ml of deionized water and stir to dissolve the boric acid. After continuing to stir for 30 minutes, a transparent solution with a concentration of 0.5 mol / L was obtained. Under the condition of continuous stirring, add 9.80g of sodium azide, continue stirring to make it all dissolve. Add 2.86ml of hydrazine hydrate while stirring to obtain a transparent homogeneous solution. The above mixed solution was transferred to a reaction kettle, and water was added to make ...
Embodiment 2
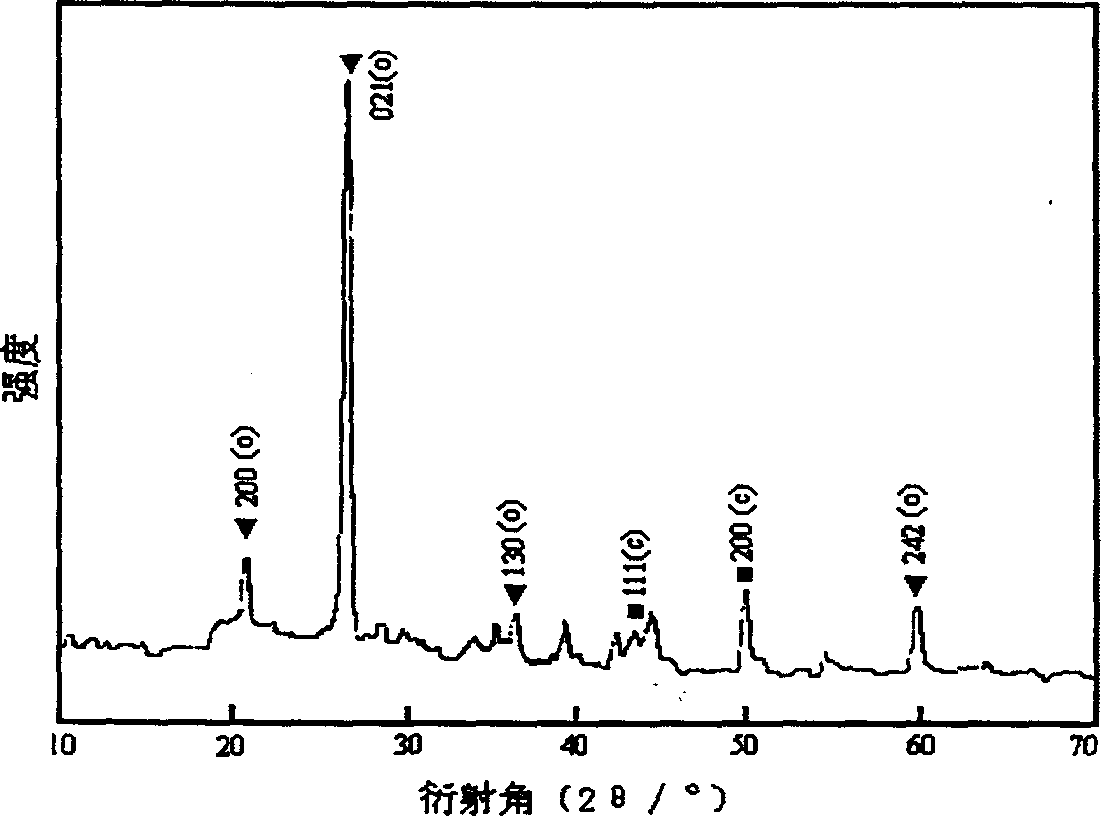
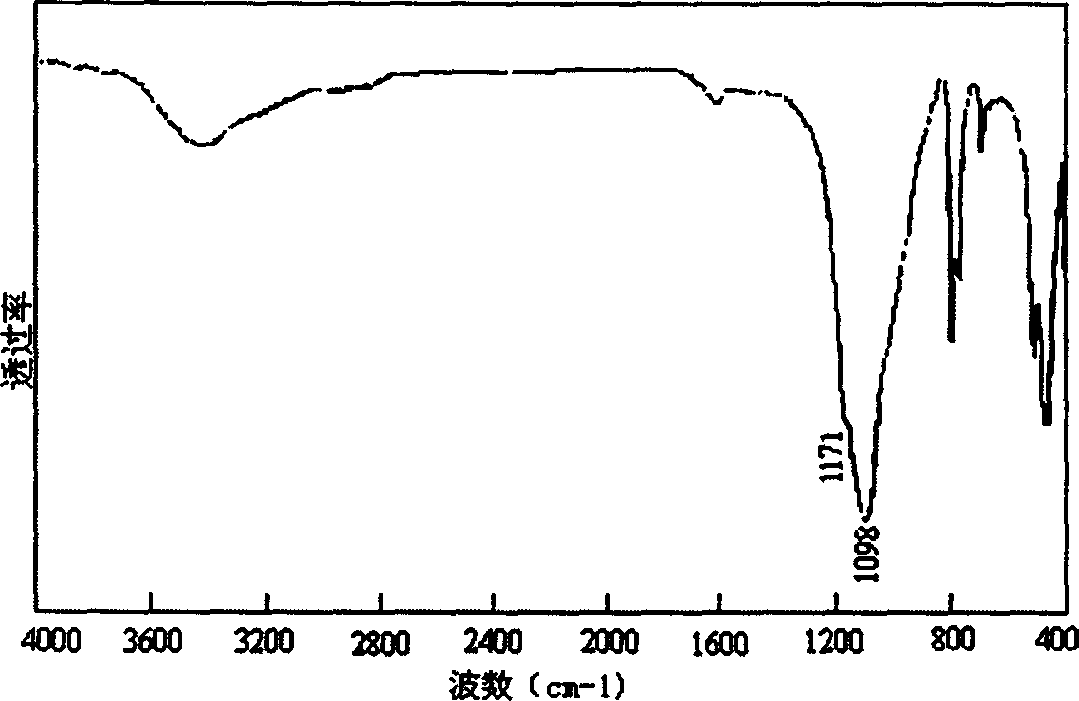
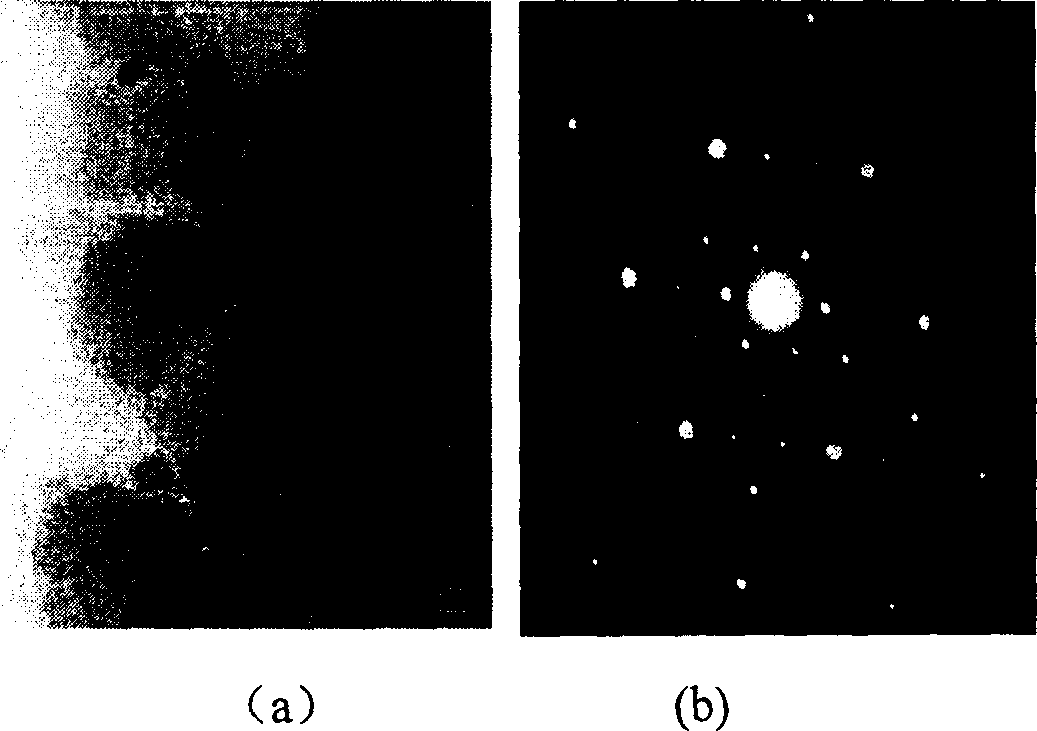
[0052] figure 1 is the X-ray diffraction pattern (XRD) of the above sample, figure 2 is the infrared absorption spectrum diagram of the obtained BN sample. image 3 It is the transmission electron microscope photo and the corresponding electron diffraction pattern of the sample prepared in Example 1. The particle size of the sample is about 300nm, and the electron diffraction spots are obtained by the incident electron beam along the cubic BN[111] crystal zone axis. Embodiment 2. prepare BN nanopowder
[0053] Concrete operation process is the same as embodiment 1, and difference is: replaced 9.8g NaN with 6.8g trimethylamine in the present embodiment 3 , and the filling ratio of the reactor was 30%, the temperature of the reaction system was rapidly raised to 400° C. (50° C. / minute), and the total reaction was 24 hours, and the rest remained unchanged.
[0054] Figure 4 is the X-ray diffraction pattern (XRD) of the BN sample prepared by the rapid heating method. There ...
Embodiment 3
[0055] Embodiment 3. prepare BN micron powder
[0056] The types and proportions of the raw materials used are exactly the same as in Example 1, except that the filling rate of the reactor is 60% in this example, and the reaction temperature is raised to 220°C at a rate of 0.2-0.4°C / min for 144 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com