Method for producing indigo
A production method and technology for indigo, applied in the field of condensation and hydrolysis reaction of the first two parts in the production of indigo, can solve problems such as restricting the development of production enterprises, difficult disposal of waste residues, affecting environmental protection, etc., to simplify production processes and reduce energy consumption. , the effect of pure color light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
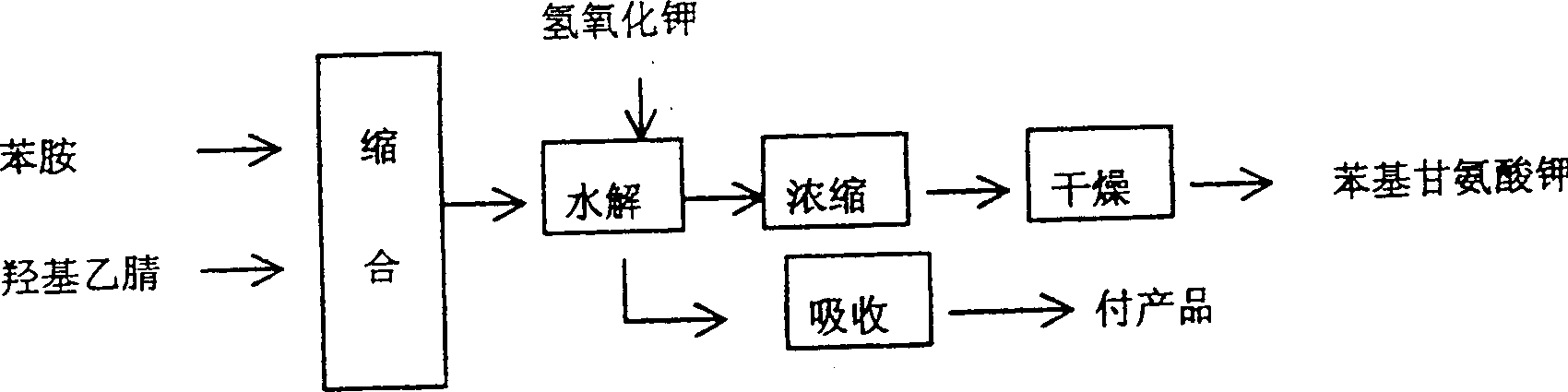
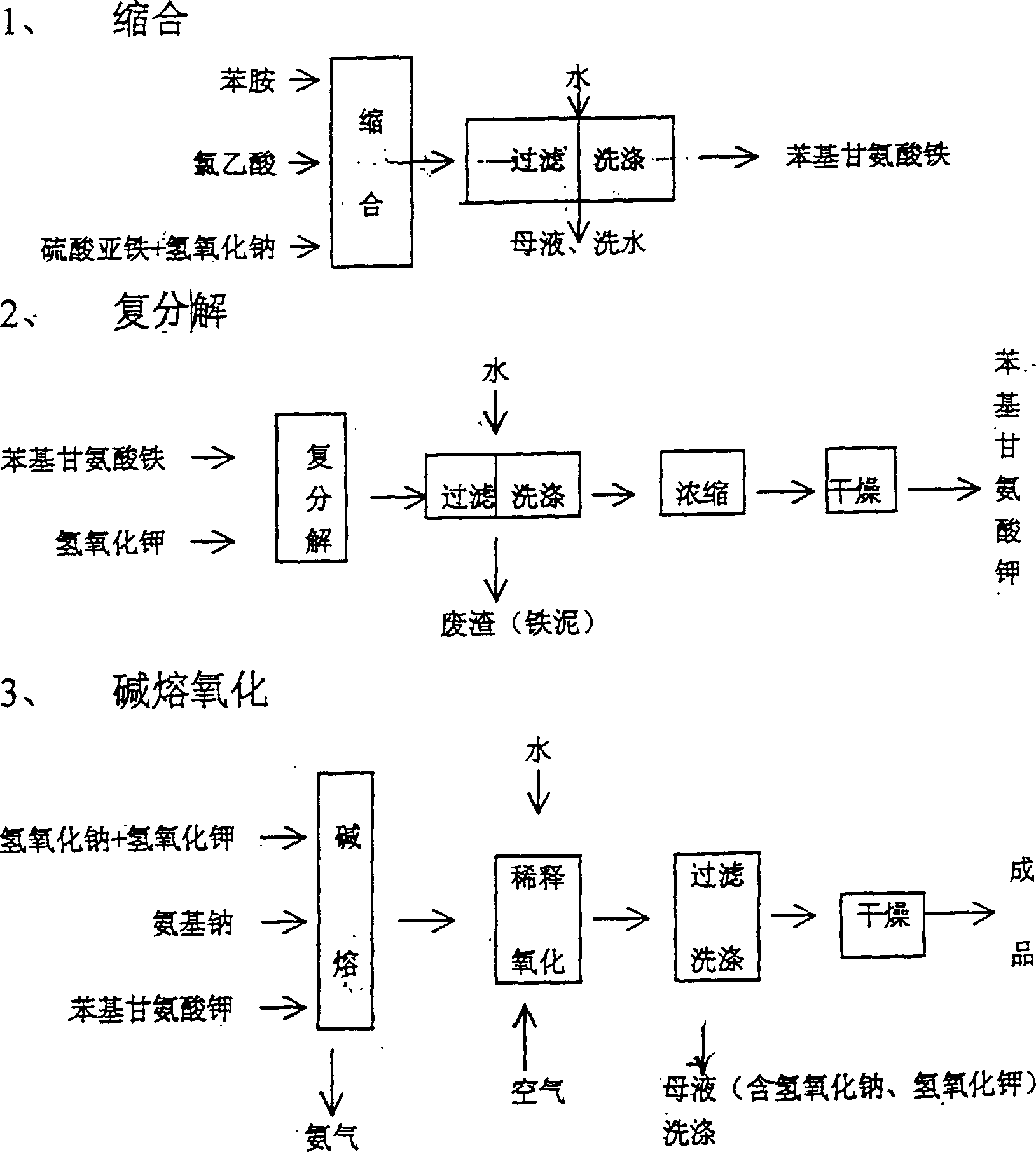
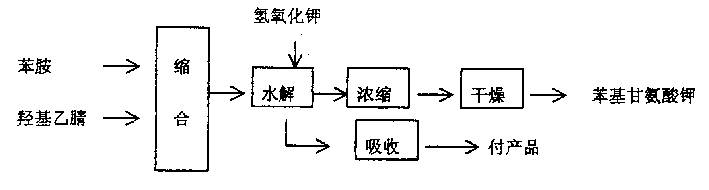
[0013] Put aniline and hydroxyacetonitrile in the condensation reactor with a molar ratio of 1:0.9, and react at 65 degrees Celsius. After 5 hours of reaction, all the reaction materials are put into the hydrolysis reactor, and potassium hydroxide The same molar ratio as aniline is put into the hydrolysis reactor, and the hydrolysis reaction is carried out under boiling and reflux, and the ammonia gas discharged from the reaction is absorbed by dilute sulfuric acid until the ammonia gas is no longer discharged in the hydrolysis reactor, and finally the The obtained reaction material is concentrated, dried to obtain white powdery potassium phenylglycinate, and then the potassium phenylglycinate and sodium hydroxide plus potassium hydroxide and sodium amide enter the alkali fusion oxidation reaction. About the alkali fusion oxidation reaction and the prior art The same, not described in detail here, the following
[0014] Embodiment no longer illustrates.
Embodiment 2
[0016] Use a metering tank to place aniline and hydroxyacetonitrile in the condensation reactor at a molar ratio of 1:1, react at 85 degrees Celsius, and after 4 hours of reaction, put all the reaction materials of aniline and hydroxyacetonitrile into hydrolysis Reactor, and potassium hydroxide is slowly put into the hydrolysis reactor with the same molar ratio of aniline, and the hydrolysis reaction is carried out under boiling and reflux, and the ammonia gas discharged from the reaction is absorbed by dilute hydrochloric acid until it is in the hydrolysis reactor Until the ammonia gas is no longer discharged, the obtained reaction materials are finally concentrated and dried to obtain slightly yellow powdered potassium phenylglycinate, and then the potassium phenylglycinate, sodium hydroxide, potassium hydroxide and sodium amide are put into alkali fusion oxidation reaction.
Embodiment 3
[0018] Use a metering tank to place aniline and hydroxyacetonitrile in the condensation reactor at a molar ratio of 1:1, react at 85 degrees Celsius, and after 4 hours of reaction, put all the reaction materials of aniline and hydroxyacetonitrile into hydrolysis Reactor, slowly put potassium hydroxide into the hydrolysis reactor in the same molar ratio as aniline, carry out the hydrolysis reaction under boiling and reflux, and absorb the ammonia gas discharged from the reaction with dilute phosphoric acid until it is in the hydrolysis reactor Until the ammonia gas is no longer discharged, the obtained reaction materials are concentrated and dried to obtain slightly yellow flaky potassium phenylglycinate, and then the potassium phenylglycinate, sodium hydroxide, potassium hydroxide and sodium amide are put into alkali fusion oxidation reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com