ELectroplated aluminium parts and process of production thereof
A component, multi-layer electroplating technology, used in the electroplating of currency blanks, electroplated aluminum parts, and coinage products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1 has the electroplating currency of copper plating layer
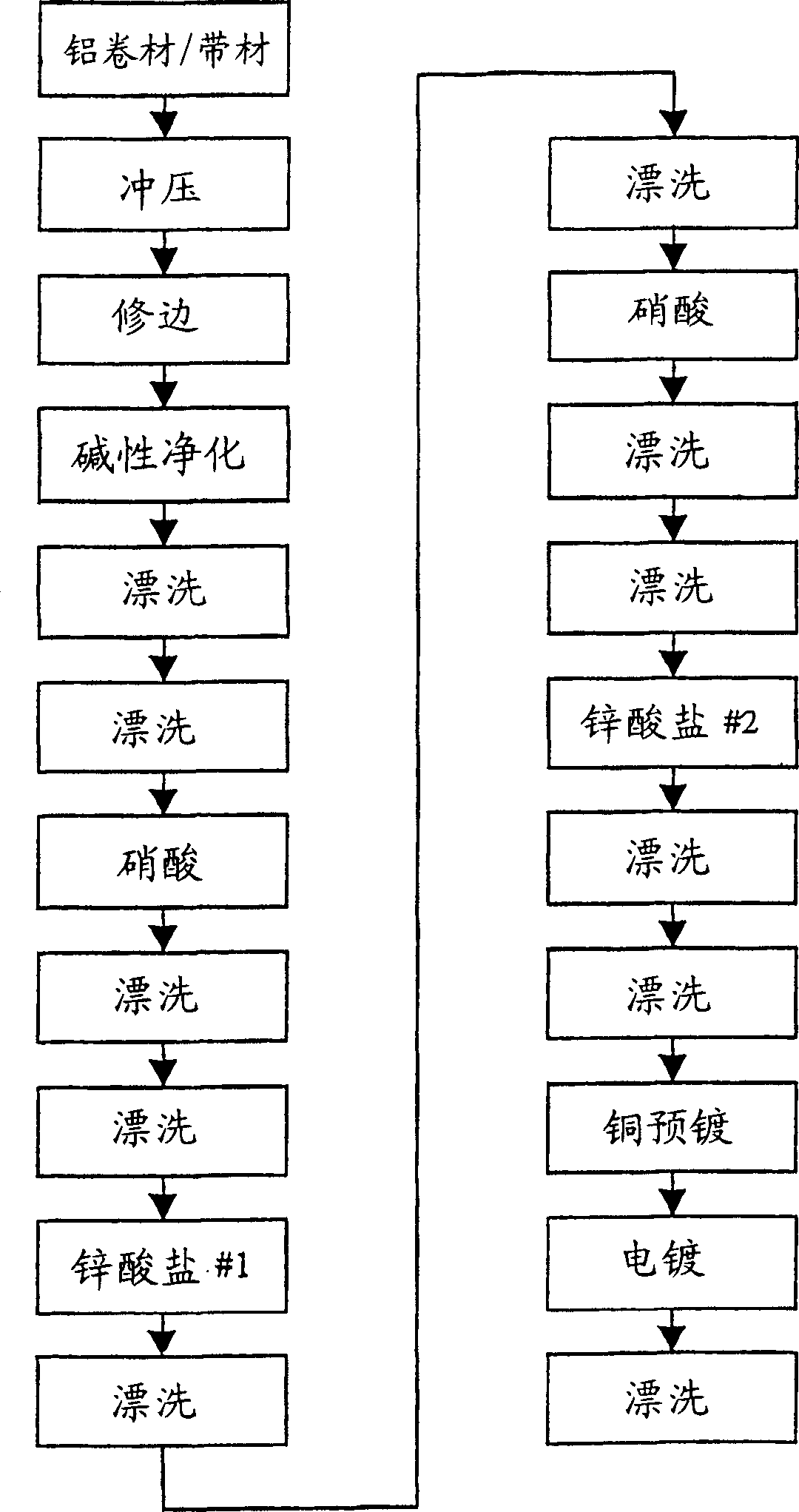
[0090] A 5052 gauge sheet of 4 feet, 8 feet long, and 0.0625 inches thick was purchased from a supplier and cut to 8 inches wide. The 8 inch strips were fed into a Minster PM3-125 punch press to produce cores for plating. The press adopts the picture A series of punches and dies arranged in a pattern to produce a round sheet called a billet. The billet had a diameter of 20.0 mm and a core thickness of 1.5 mm.
[0091]After the stamping operation, the blank is deburred and then edged with a standard EVD type coin beading machine. The machine operates automatically, where the billets are fed into grooved wheels using a vibrating feeder with controlled feed rate into a work zone that reduces the diameter of the billet and forms an edge on the billet. The edge height produced in the edge forming operation was about 1.70 mm. After blanking, deburring and edge formation, the blank is sent to pretreatm...
Embodiment 2
[0105] Embodiment 2 Improved copper electroplating bath
[0106] To obtain a "bright" electroplating bath, this example demonstrates the use of a two-step zincating, copper preplating, and copper electroplating bath. Example 1 was followed unless otherwise stated.
[0107] Edged aluminum blanks were prepared, followed by a zincating process similar to Example 1. After zincating, the blank was immersed in the low pH sodium cyanide preplating solution of Example 1, but with a pH of 9.0, 23 gpl of free cyanide and 30 gpl of copper. The zincate-treated blanks were immersed in the pre-plating bath for 15 minutes.
[0108] The next step is to electroplate the final copper plating on the blank using copper pre-plating. The copper concentration in the electroplating bath was 25.5 gpl. The free potassium cyanide concentration was 10.2 gpl. In addition, the bath contains 0.3% by volume of Atotech TM additive CL-3, and 0.5% Atotech additive CL-4. These additive brighteners were pu...
Embodiment 3
[0110] Example 3 Nickel-plated aluminum blank
[0111] This example demonstrates the method of the invention with a nickel-plated aluminum bill blank. Except for the final electroplating step, the similar blank preparation process is the same as in Example 1. After copper preplating, the blank is dipped into an electroplating bath of nickel sulfamate. The pH value of the nickel plating bath is 2.35, the concentration of boric acid is 42.2gpl, and the surface tension is 37.6 dynes / cm 2 , the nickel concentration is 113gpl.
[0112] The blanks were plated in a nickel sulfamate plating bath for 3 hours. The blanks were then rinsed in two separate rinsing steps, each for 2 minutes, followed by coinmaking in the same manner as in Example 1. The adhesion of the coating was checked with a 90° bend test. The plating cracks along the outside diameter of the bend, but cannot be stripped from the surface with sharp tools. Referring to Example 1, this demonstrates the strong adhesio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Tube length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com