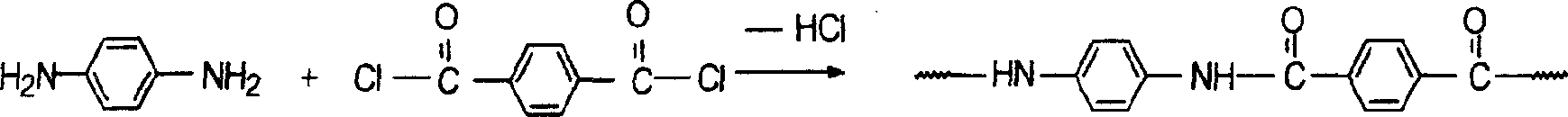Copolyaramide containing phthalazine biphenyl structure and its preparation method
A technology of naphthone biphenyl and copolyaramide is applied in the synthesis field of copolyaramide, which can solve the problems of solution direct spinning and PPTA inability, and achieve the effects of easy purification, good solubility, and simple and feasible synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
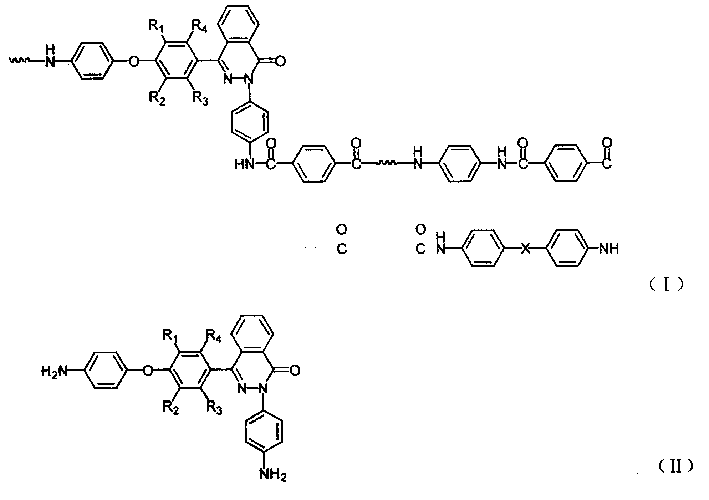
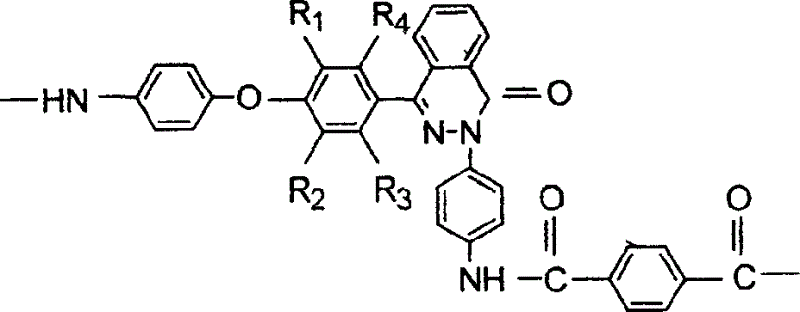
[0030] In a three-necked flask equipped with a stirrer, a reflux condenser, a thermometer, and a nitrogen inlet and outlet, under nitrogen protection, 1.5768 grams of 1,2-dihydro-2-(4-aminophenyl)-4-[4 -(4-aminophenoxy)-phenyl]-phthalazin-1-one, 1.1262 grams of 4,4'-diaminodiphenyl ether, 1.014 grams of p-phenylenediamine, a total of 18.75mmol and equivalent Terephthalic acid 3.1149g (18.75mmol), anhydrous lithium chloride 6.48g, triphenyl phosphite (TPP) 15ml, pyridine (PY) 18ml, N-methylpyrrolidone (NMP) 48ml, under nitrogen protection in After reacting at 110°C for 30 minutes, the viscosity of the reaction system increased significantly. After reacting for 6 hours, the reaction mixture was poured into 400ml of ethanol and water (1:1) to settle under vigorous stirring to obtain a white flocculent copolymer, which was filtered and washed thoroughly with hot water, and the polymer was vacuum-dried at 120°C After 24 hours, a pure copolyaramid resin was obtained. Its intrinsic...
Embodiment 2
[0032]In a three-necked flask equipped with a stirrer, a reflux condenser, a thermometer, and a nitrogen inlet and outlet, under the protection of nitrogen, add 0.3 g of lithium chloride and 1.14 ml of pyridine. When the salt is completely dissolved, add 0.6728 g of 1,2-bis Hydrogen-2-(4-aminophenyl)-4-[4-(4-aminophenoxy)-phenyl]-phthalazin-1-one, 0.3204 g 4,4'-diaminodiphenyl Ether, 0.5190 grams of p-phenylenediamine, add 0.8161 grams of terephthaloyl dichloride while stirring, after 10 minutes of reaction, add the remaining 0.8162 grams of terephthaloyl dichloride, accelerate stirring, keep the reaction temperature at 20 °C under normal pressure, and react The reaction stopped after 6 hours. The reaction mixture was poured into 400ml of ethanol and water (1:1) to settle under vigorous stirring to obtain a white flocculent polymer, which was filtered and washed thoroughly with hot water, and the polymer was vacuum-dried at 120°C for 24 hours to obtain pure Copolyaramid resin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com