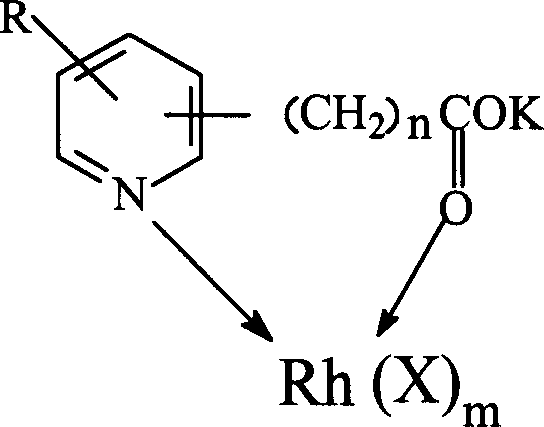
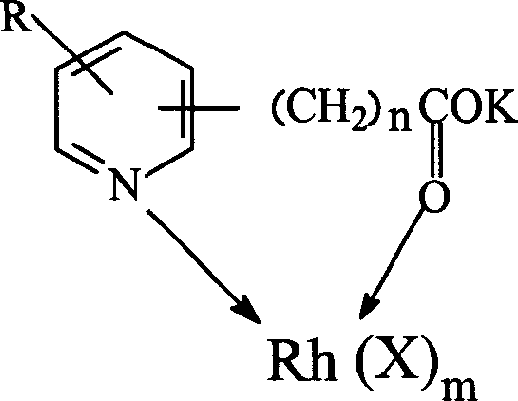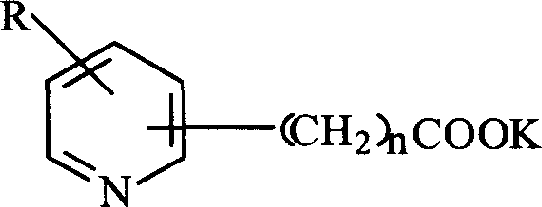Catalytic system used for homogeneous hydroxylation reaction and its manufacturing method and application
A catalytic system, technology of carbonylation reaction, applied in the direction of carbon monoxide reaction to prepare carboxylic acid, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of consumption of large promoter methyl iodide, catalyst solubility Not good, unable to achieve industrialization and other problems, to achieve good catalytic activity and stability, reduce the formation of precipitates, and coordinate stable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 0.02 moles of pyridine-2-carboxylic acid and potassium hydroxide monohydrate and dissolve them in methanol, methanol is 0.5 mol, react for 1 hour under stirring, precipitate with excess ether, filter, wash, and dry to obtain pyridine-2 - Potassium formate.
[0036] In 0.5 mol of methanol containing 0.02 mol of potassium pyridine-2-formate, add dropwise a methanol solution containing 0.02 mol of rhodium acetate, wherein methanol is 0.7 mol, after the dropwise addition, continue to stir for 20 minutes, precipitate with excess ether, Filter and dry to constant weight to obtain bimetallic catalyst.
Embodiment 2
[0038] Weigh 0.01mol of pyridine-3-acetic acid and potassium hydroxide monohydrate, dissolve in 0.4mol of methanol, react for 1 hour under stirring, precipitate with excess ether, filter, wash, and dry to obtain pyridine-3-acetic acid potassium.
[0039] In 0.2 mol of methanol containing 0.01 mol of potassium pyridine-3-acetate, dropwise add an aqueous solution containing 0.01 mol of rhodium nitrate, wherein the water is 0.8 mol, after the dropwise addition, continue stirring for 20 minutes, precipitate with excess ether, and filter , and dried to constant weight to obtain a bimetallic catalyst.
Embodiment 3
[0041] Weigh 0.025mol of pyridine-3-propionic acid and potassium hydroxide monohydrate and dissolve them in 0.8mol of methanol, react for 1 hour under stirring, precipitate with excess ether, filter, wash, and dry to obtain pyridine-3-propane Potassium acid.
[0042] In 0.2 mol of methanol containing 0.025 mol of potassium pyridine-3-acetate, add dropwise a methanol solution containing 0.025 mol of rhodium acetate, in which 1 mol of water, after the dropwise addition, continue to stir for 20 minutes, use excess ether to precipitate and filter , and dried to constant weight to obtain a bimetallic catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com