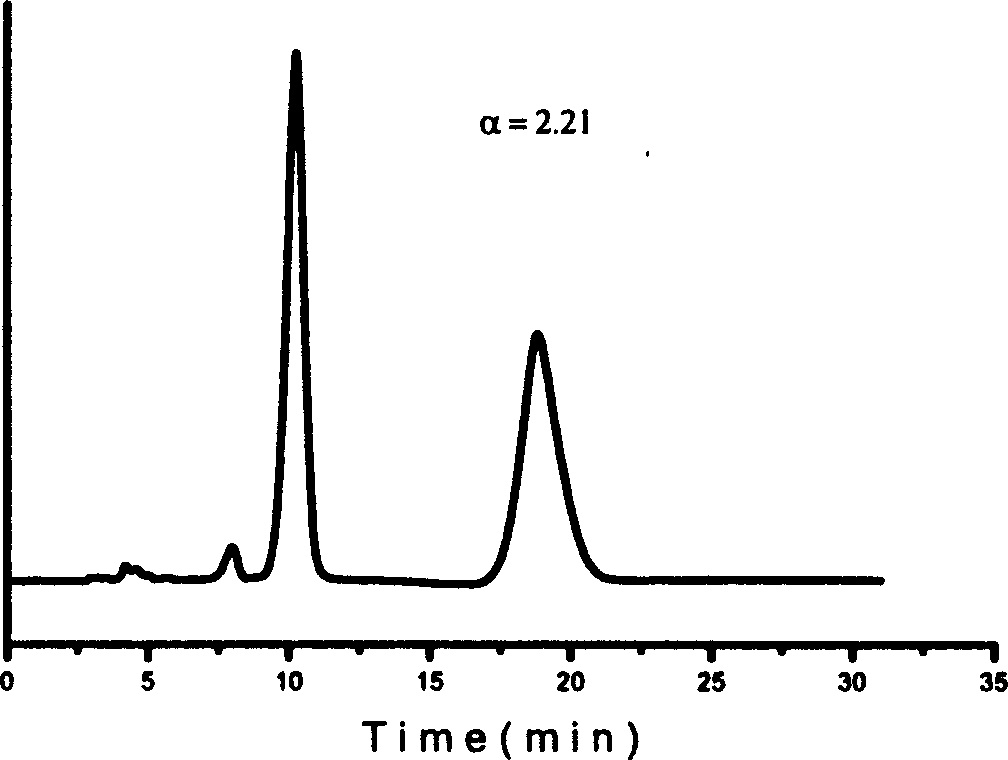
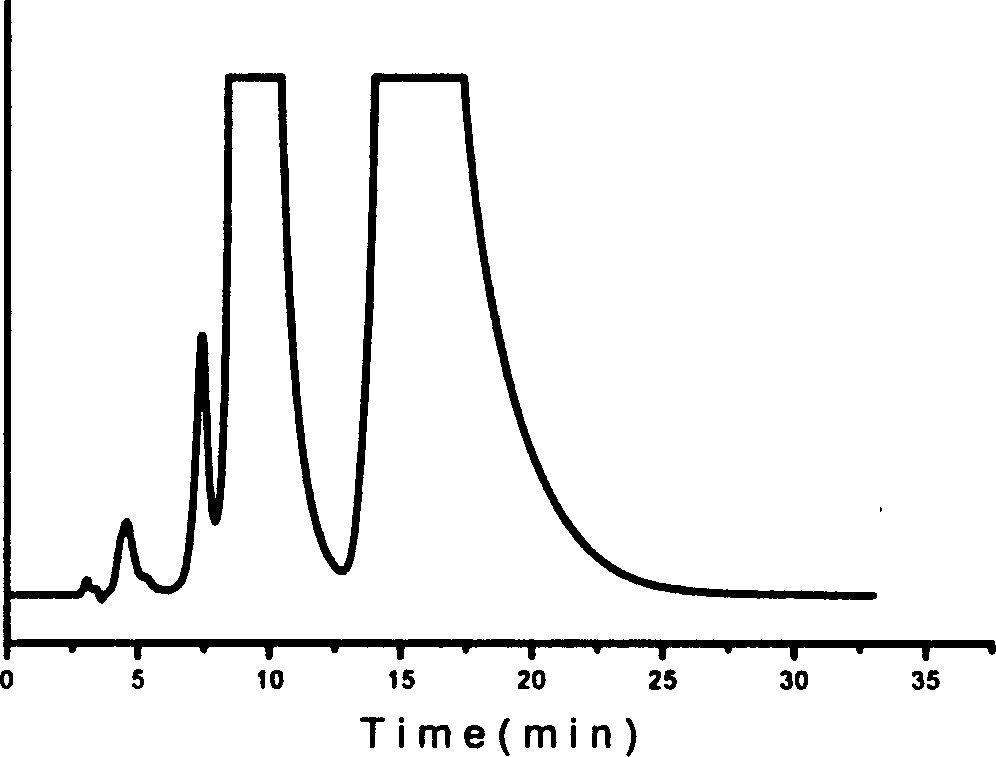
Method for splitting antipode of 3-butylbenzene phthalein
A technology of n-butylphthalide and enantiomers, which is applied in the production field of optically active 3-n-butylphthalide, can solve the problem of unsatisfactory optical purity of optically active products, tedious and time-consuming separation process, and narrow scope of application of the method, etc. problem, to achieve the effect of reliable measurement results, good splitting effect and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Preparation of chiral stationary phase:
[0023] ① Prepare γ-aminopropyl silica gel according to conventional methods;
[0024] ② Synthesis of cellulose-tris(3,5-dimethylphenylcarbamate) derivatives: Take 1.0 g of dry cellulose in a three-necked flask, add 50 mL of dry pyridine, heat to reflux for 24 hours, then add 5 mL of 3, 5-Dimethylphenylisocyanate, electromagnetic stirring reflux 24; cooling, slowly add a large amount of methanol, there is solid precipitation, filter, the solid product is washed with methanol, vacuum-dried, the final product is a white solid; yield 85 %;
[0025] ③ Dissolve 0.5g of cellulose-tris(3,5-dimethylphenylcarbamate) derivative in 25ml of tetrahydrofuran, add the above solution into 2.0g of aminopropyl silica gel, stir and evaporate until tetrahydrofuran evaporates to dryness , to finally obtain a cellulose-tri(3,5-dimethylphenylcarbamate) chiral stationary phase with a coating amount of 20% (mass fraction);
[0026] 2) Chiral column...
Embodiment 2
[0029] 1) Preparation of chiral stationary phase:
[0030] ① Prepare γ-aminopropyl silica gel according to conventional methods;
[0031] ②Synthetic starch (amylose or amylopectin) phenyl carbamate derivatives: Take 1.0 g of dry starch in a three-neck flask, add 50 mL of dry pyridine, heat and reflux for 24 hours, then add 5 mL of phenyl isocyanate, and stir with electromagnetic Reflux for 24°C; cool down, slowly add a large amount of methanol, then a solid precipitates, filter, wash the solid product with methanol, and dry it in vacuum, the final product is a white solid; the yield is 85%.
[0032] ③Dissolve 0.5g of starch phenylcarbamate derivatives in 25ml of chloroform, add the above solution into 2.0g of aminopropyl silica gel, stir and evaporate until tetrahydrofuran volatilizes to dryness, and finally obtain a coating amount of 20% (mass fraction ) starch phenyl carbamate chiral stationary phase;
[0033] 2) Chiral column packing: take 2.0 g of starch phenylcarbamate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com