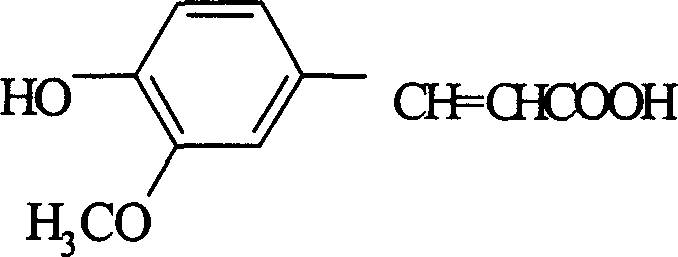
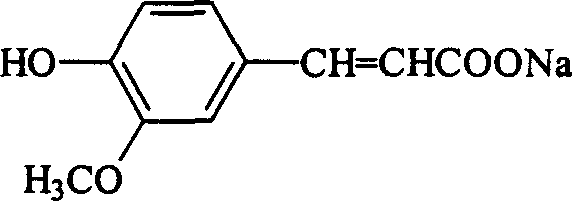
Ferulic acid and ferulate preparation method
A technology of ferulic acid and dimethyl malonate, which is applied in the direction of carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of complex components, high cost of malonate, and high cost. Achieve simple reaction steps, stable properties, and prevent discoloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
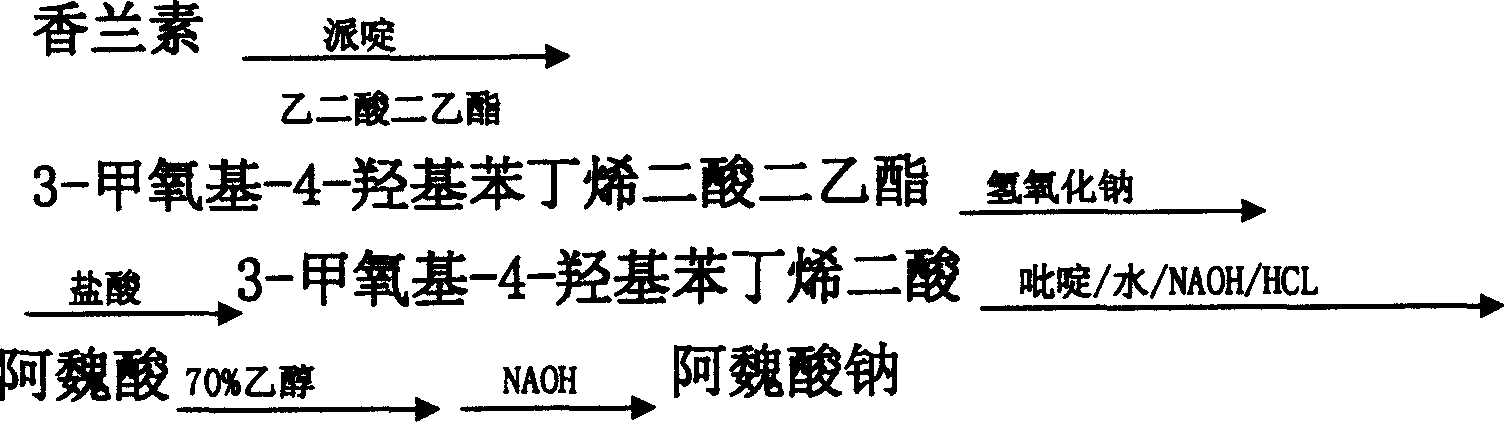
[0049] 1, Preparation of 3-methoxy-4-hydroxybenzenedioic acid diethyl ester
[0050] Raw material ratio:
[0051] Vanillin: diethyl malonate: piperidine = 1: 1.23: 0.043
[0052] Put 184.5 kg of accurately measured diethyl malonate in a dry glass-lined reaction pot, start stirring, put in 150 kg of measured vanillin, heat up to about 30-40°C, stop heating, and put in 6.45 kg of piperidine kg, and the temperature is raised naturally, when it rises to 45°C, the reaction time is kept for 8 to 12 hours. After the reaction is completed, put the material into a stainless steel barrel, crystallize naturally, place it overnight, crystallize for more than 12 hours, centrifuge, apply the mother liquor, wash the filter cake with drinking water, and dry it to obtain the condensate 3-methoxy-4-hydroxy Diethyl phthalenate. Dried to 273 kg for saponification. The yield is 94.09%.
[0053] Using a method similar to the above, 3-methoxy-4-hydroxybenzene dioic acid dimethyl and 3-methoxy-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com