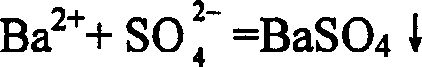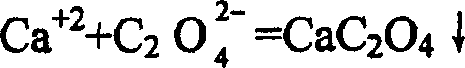Purification process of removing impurity sodium from lithium chloride
A purification method and technology of lithium chloride, applied in the direction of alkali metal chloride, alkali metal halide purification, etc., can solve the problems of aggravating the difficulty of filtration and separation, long soaking and stirring time, unfavorable actual production, etc., achieving low cost, Easy operation and strong compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
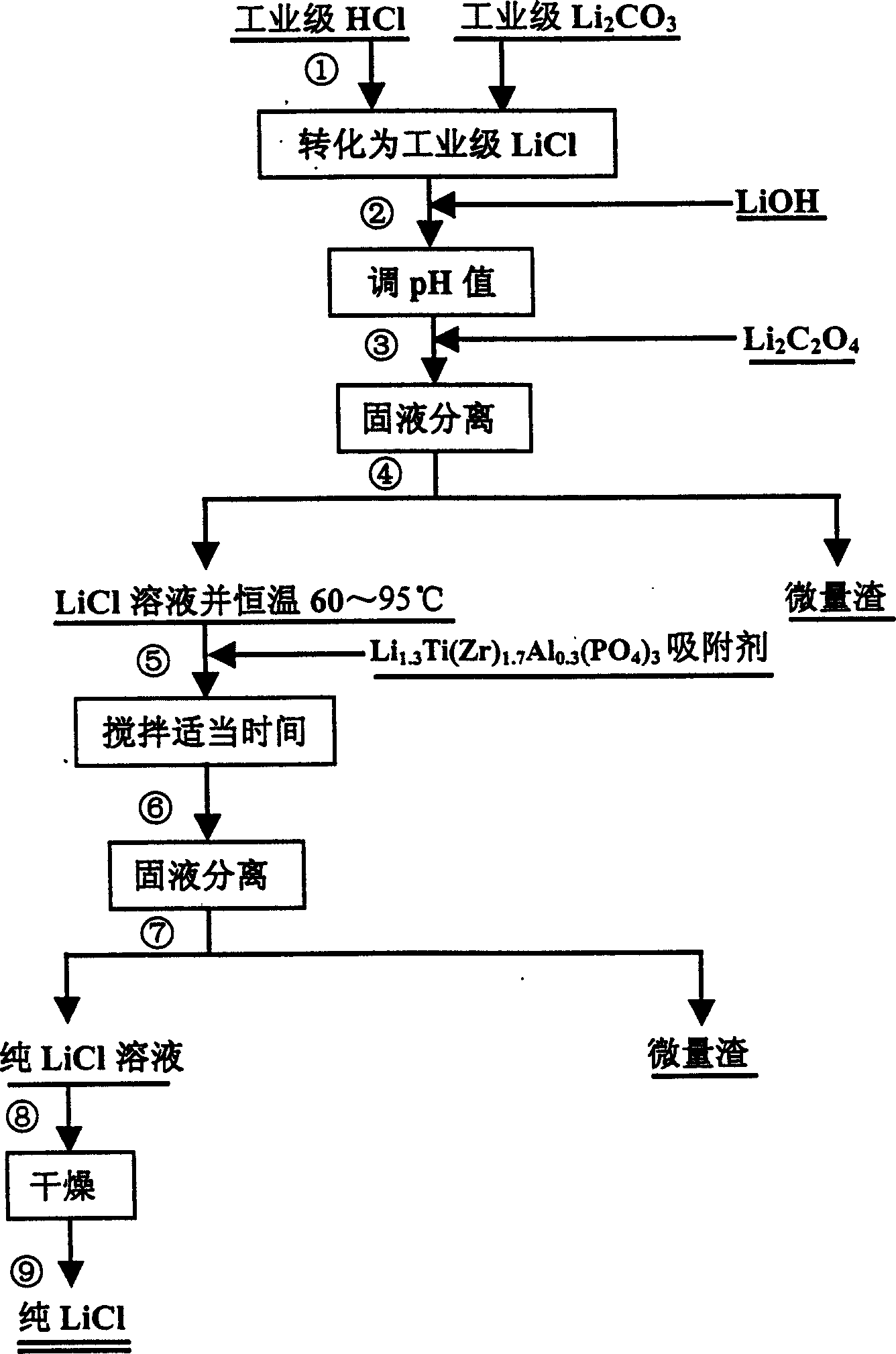
[0026] Example 1: Place a beaker containing 300 milliliters of industrial hydrochloric acid (concentration 30-33%) on a magnetic stirrer for magnetic stirring, then slowly add industrial-grade lithium carbonate (about 100 grams), monitor with pH test paper, close to neutral until sex. After the neutralization reaction was completed, LiOH was added to adjust the pH value to about 11, and then 10 ml of lithium oxalate with a concentration of 2.5% was added. Filtrate with a 0.5 μm microfiltration membrane to obtain the lithium chloride supernatant. Add 2.5 grams of Li 1.3 Ti 1.7 al 0.3 (PO 4 ) 3 The powder (average particle size is 3.5 microns), respectively stirred slowly for 5 hours, and then filtered with a 0.5 micron microfiltration membrane. The filtered lithium chloride liquid was placed in a titanium crucible and dried in an oven, and samples were taken for testing. The results of the atomic absorption spectrophotometer test show that the sodium (Na) impurities in t...
example 2
[0027] Example 2: Place a beaker with 300 milliliters of industrial hydrochloric acid (concentration 30-33%) on a magnetic stirrer for magnetic stirring, then slowly add industrial-grade lithium carbonate (about 100 grams), monitor with pH test paper, close to neutral until sex. After the neutralization reaction was completed, LiOH was added to adjust the pH value to about 11, and then 10 ml of lithium oxalate with a concentration of 2.5% was added. Filtrate with a 0.5 μm microfiltration membrane to obtain the lithium chloride supernatant. Add 5 grams of Li 1.3 Ti 1.7 al 0.3 (PO 4 ) 3 The powder (average particle size is 3.5 microns), respectively stirred slowly for 10 hours, and then filtered with a 0.5 micron microfiltration membrane. The filtered lithium chloride liquid was placed in a titanium crucible and dried in an oven, and samples were taken for testing. The results of the atomic absorption spectrophotometer test show that the sodium (Na) impurities in lithium ...
example 3
[0028] Example 3: Place a beaker with 300 milliliters of industrial hydrochloric acid (concentration 30-33%) on a magnetic stirrer for magnetic stirring, then slowly add industrial-grade lithium carbonate (about 100 grams), monitor with pH test paper, close to neutral until sex. After the neutralization reaction was completed, LiOH was added to adjust the pH value to about 11, and then 10 ml of lithium oxalate with a concentration of 2.5% was added. Filtrate with a 0.5 μm microfiltration membrane to obtain the lithium chloride supernatant. Add 5 grams of Li to 300 milliliters of lithium chloride clear solution at a constant temperature of 75 1.3 Ti 1.7 al 0.3 (PO 4 ) 3 Powder (average particle size 3.5 microns), stirred slowly for 10 hours, and then filtered with 0.5 micron microfiltration membrane. The filtered lithium chloride liquid was placed in a titanium crucible and dried in an oven, and samples were taken for testing. The result of testing with an atomic absorpt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com