Liquid phase synthesis process of oxime strain ester by poly-ethandiol
A technology of polyethylene glycol ester and polyethylene glycol, applied in the field of preparation of organic compound pesticides, can solve problems such as synthesis without using tristrobin, and achieve the effect of simplifying the steps of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
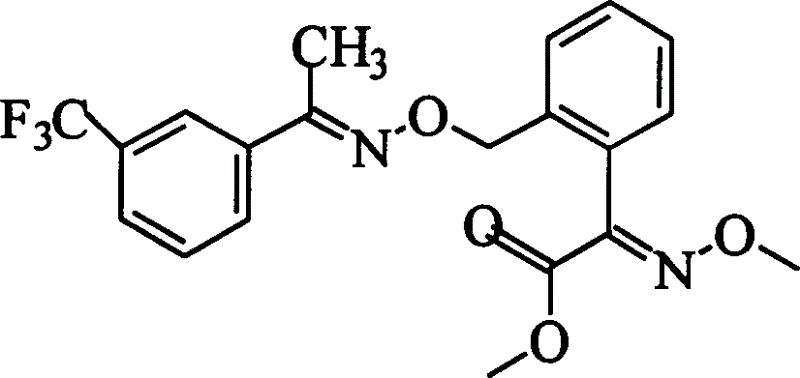
[0017] 1. Polyethylene glycol 2-(2'-methylphenyl)-2-carbonyl acetate
[0018] Add 2g (0.01mol) 2-(2,-methylphenyl)-2-carbonylacetic acid and 5ml thionyl dichloride into a 100ml round bottom flask equipped with a stirring and reflux condenser. Stir at room temperature for 5 hours, then rotate and evaporate. Add a small amount of thionyl chloride, add a small amount of dry n-heptane, rotate to evaporate the n-heptane, add 20ml of dichloromethane and 10g of polyethylene glycol to the flask, react at room temperature for 8 hours, distill the dichloromethane, add Diethyl ether, 2-(2'-methylphenyl)-2-carbonyl acetate polyethylene glycol ester is precipitated, dissolved in dichloromethane, dichloromethane is evaporated, and diethyl ether is added to obtain 9.8 g of relatively pure 2-( 2'-Methylphenyl)-2-carbonyl acetate polyethylene glycol ester.
[0019] 2. Synthesis of 2-(2'-methylphenyl)-2-carbonyl acetate polyethylene glycol ester-O-methyl ketoxime
[0020] Add 9.8g 2-(2'-methylpheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com