Low-temperature fuel cell platinum-tin base anode catalyst
A technology of fuel cells and catalysts, which is applied in the directions of battery electrodes, catalyst activation/preparation, physical/chemical process catalysts, etc. It can solve the problems of poor electrochemical oxidation reaction activity and achieve uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
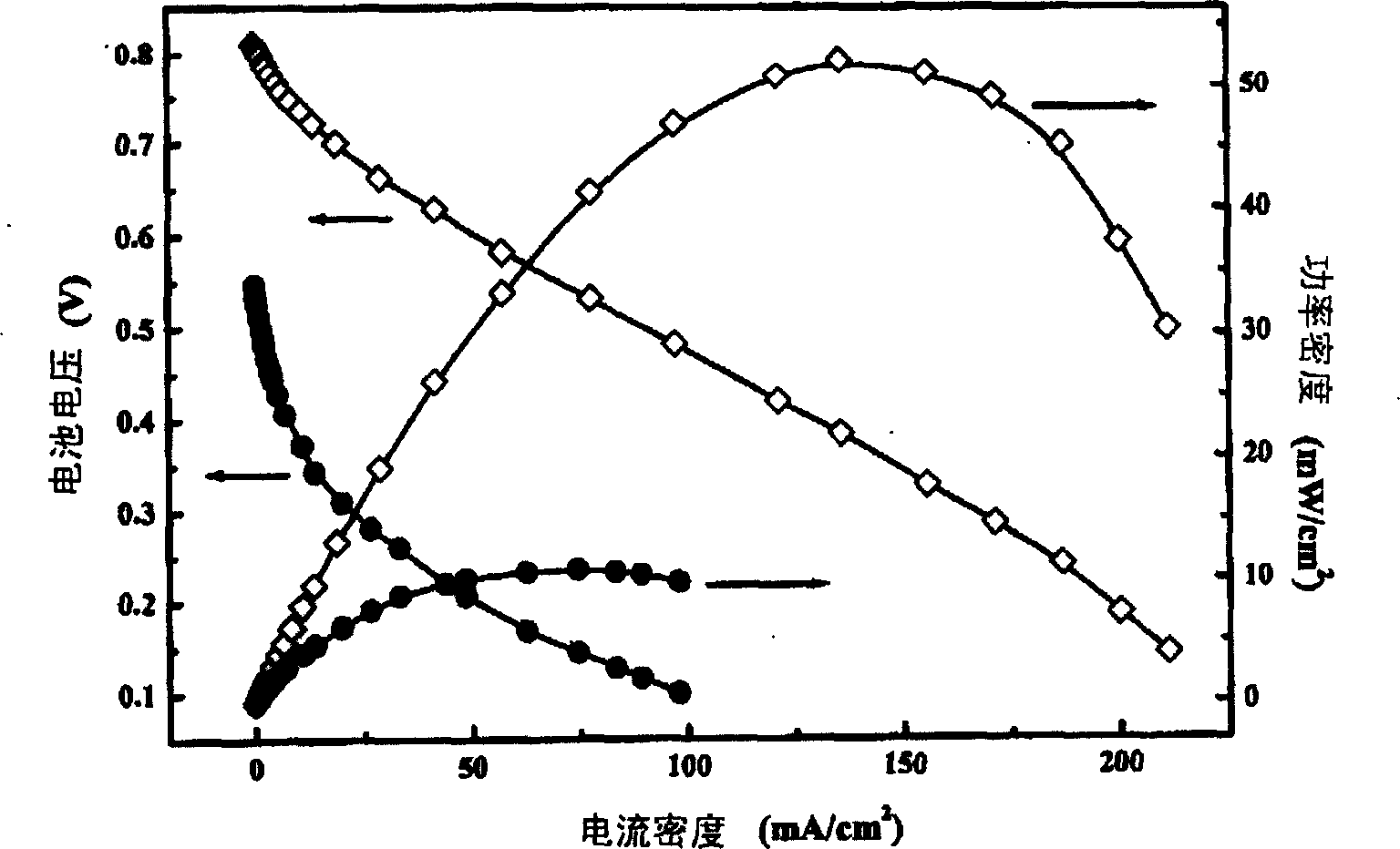
[0045] Catalyst Preparation Example 1: Preparation of Platinum Tin Carbon (PtSn / C) (32PtSn%, Pt / Sn=1) Catalyst
[0046] Weigh 2 grams of activated carbon XC-72R and disperse it with 150 ml of ethylene glycol for 30 minutes with ultrasonic vibration to prepare carbon slurry. Measure 20 ml of chloroplatinic acid / ethylene glycol solution (29.5 mg platinum / ml) and 10 ml of tin tetrachloride ethanol solution (35 mg tin / ml) to mix, ultrasonically oscillate for 20 minutes and then drop into the carbon slurry After stirring for 4 hours with argon deoxygenation, 15 ml of 1.5 mol / L sodium hydroxide / ethylene glycol solution was added dropwise, and the temperature was raised to 130°C for 4 hours after continuing to stir for 4 hours, then cooled to 25°C, and used 1.5 Mole / liter of dilute hydrochloric acid solution to adjust the pH value to 2.5, after stirring for 6 hours, filter and wash, and vacuum dry overnight at 70°C. A catalyst of 20 wt.% platinum (Pt)-12 wt.% tin (Sn) was obtained. ...
preparation Embodiment 2
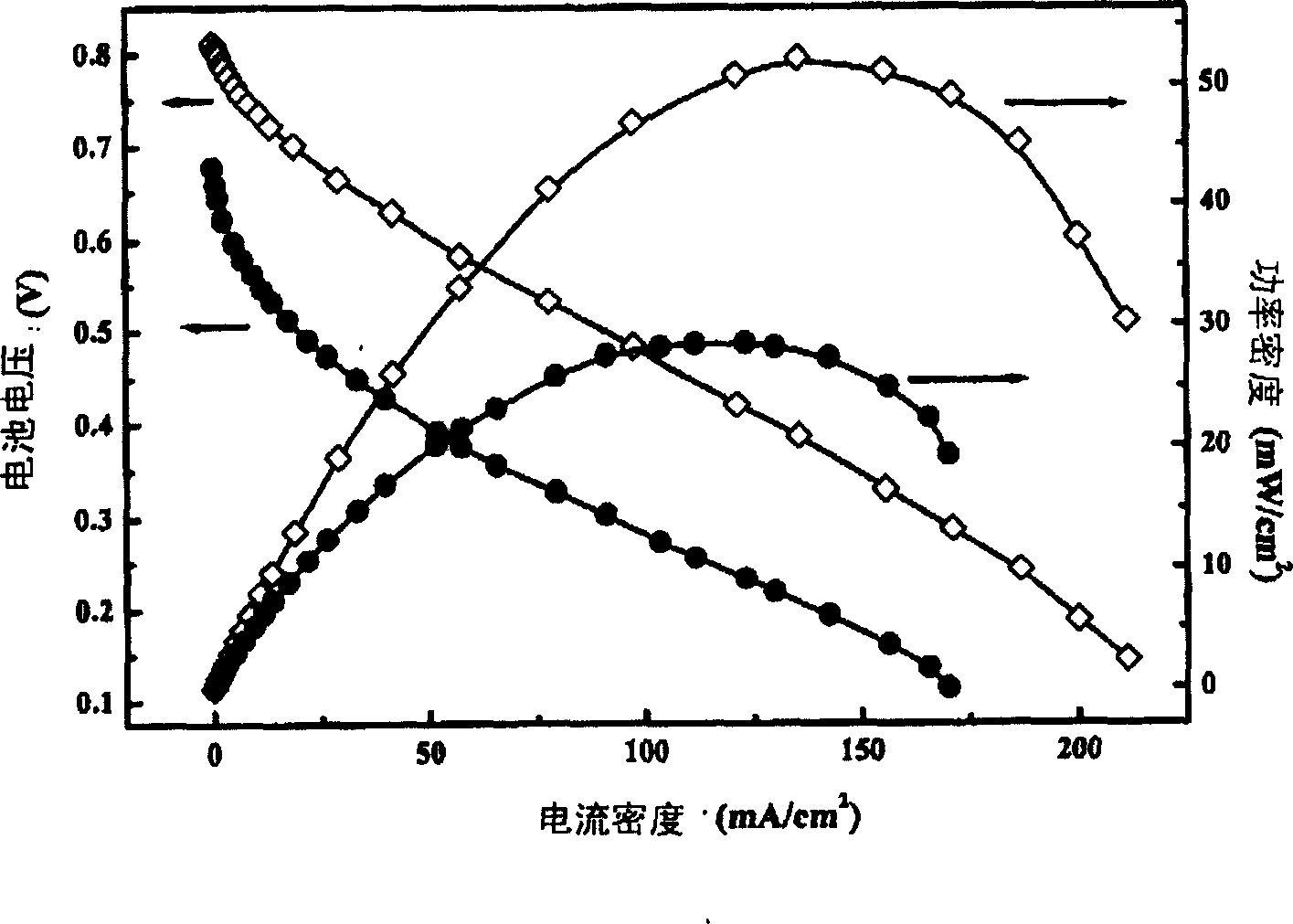
[0047] Catalyst Preparation Example 2: Preparation of Platinum Tin Ruthenium Carbon (PtSnRu / C) (42PtRu%, Pt / Sn / Ru=1) Catalyst
[0048] Activated carbon XC-72R was pre-treated with 5mol / L nitric acid solution, dried at 200°C for 4 hours, weighed 5.8 grams and dispersed with 400 ml of ethylene glycol for 30 minutes by ultrasonic oscillation to prepare carbon slurry. 5.4 grams of chloroplatinic acid (containing 2.0 grams of platinum) were dissolved in 50 milliliters of ethylene glycol, and 2.7 grams of ruthenium trichloride (containing 1.0 grams of ruthenium) were dissolved in 50 milliliters of tin chloride hydrochloric acid dilute solution (containing 1.2 grams of tin). A mixed solution of tin and ruthenium is formed. Add the tin-ruthenium mixed solution dropwise to the platinum solution, oscillate ultrasonically for 20 minutes and then transfer it to the carbon slurry. After argon deoxygenation and stirring for 5 hours, adjust the pH with 1 mol / L sodium hydroxide / ethylene glyco...
preparation Embodiment 3
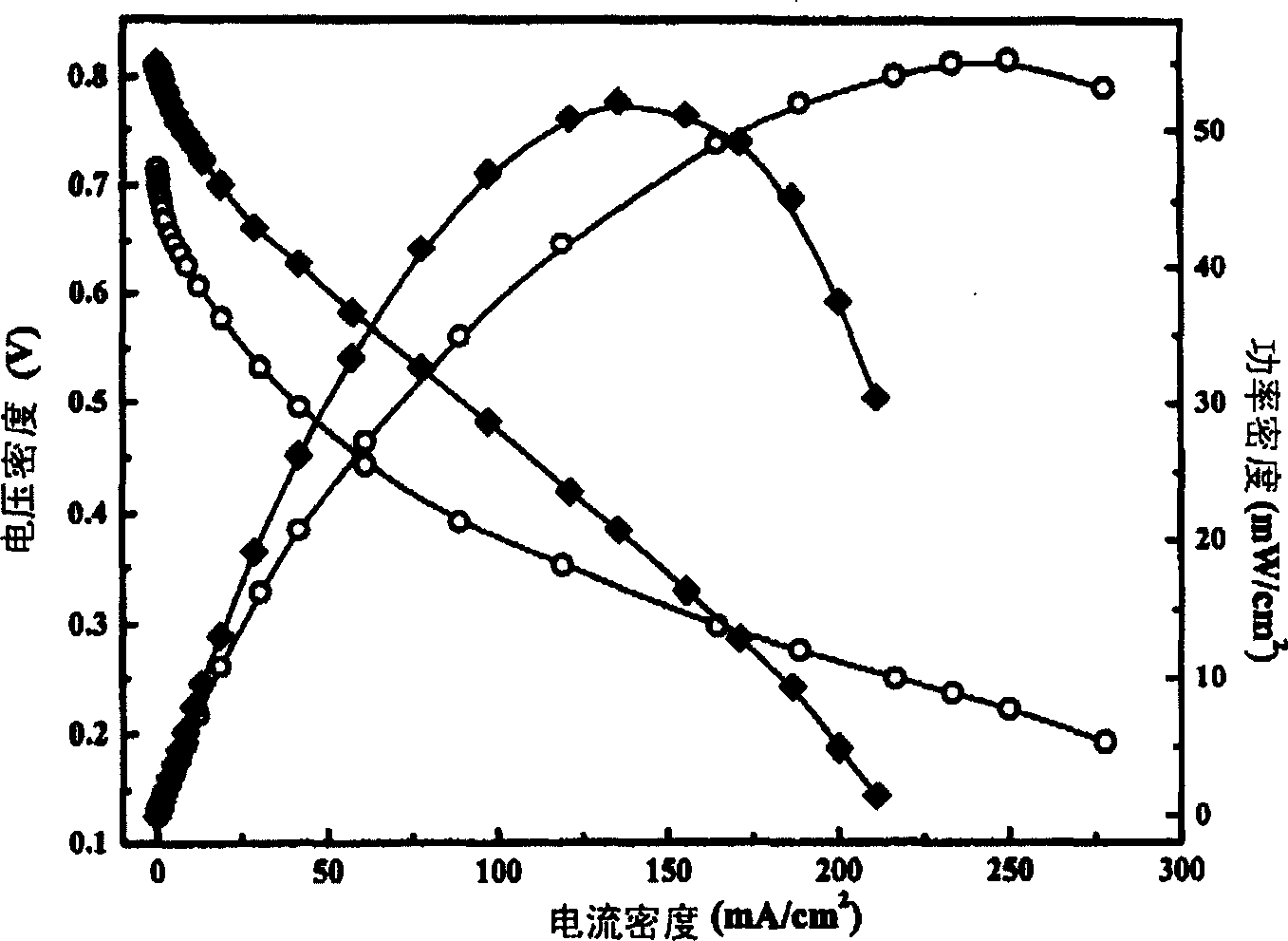
[0049] Catalyst Preparation Example 3: Preparation of Platinum Tin Carbon (PtSn / C) (26 PtSn%, Pt / Sn=2) Catalyst
[0050] A 21wt.% platinum-carbon (Pt / C) catalyst was used as a carrier, and then tin was loaded on it. Weigh 2 g of 21 wt.% platinum on carbon (Pt / C) and shake and disperse with 100 ml of ethylene glycol for 30 minutes to obtain a slurry mixture. Take 10 ml of ethanol solution of tin tetrachloride (12.7 mg tin / ml) and add it to the slurry mixture, deoxygenate with argon and stir for 2 hours, adjust the pH value to 13.5 with 1.0 mol / liter sodium hydroxide ethanol solution, and heat up to 120°C Keep it for 1 hour, then lower it to room temperature, add 150 ml of deionized water, adjust the pH value to 3 with dilute hydrochloric acid solution, stir for 1 hour, filter and wash, and vacuum dry overnight at 80°C. A catalyst of 20 wt.% platinum (Pt)-6 wt.% tin (Sn) was obtained. The results of transmission electron microscope and X-ray diffraction experiments show that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com