Method for preparing 3-hydroxypropionaldehyde by acrylaldehyde hydration
A technology for hydration of acrolein and hydroxypropionaldehyde, applied in the preparation of carbon-based compounds, preparation of organic compounds, chemical instruments and methods, etc., can solve problems such as separation difficulties, increased process complexity, and impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
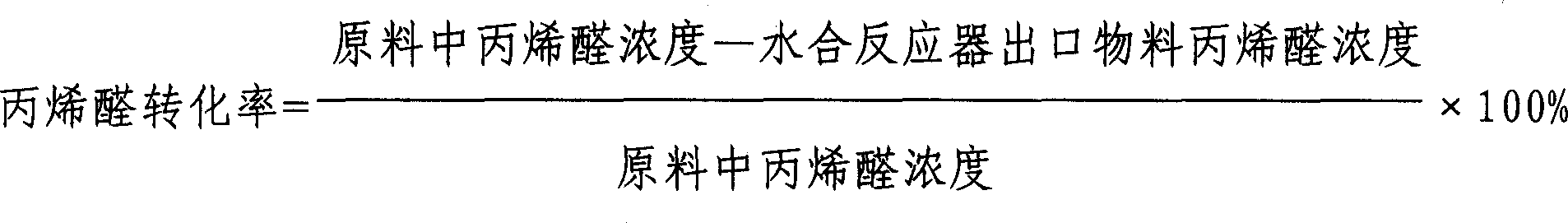
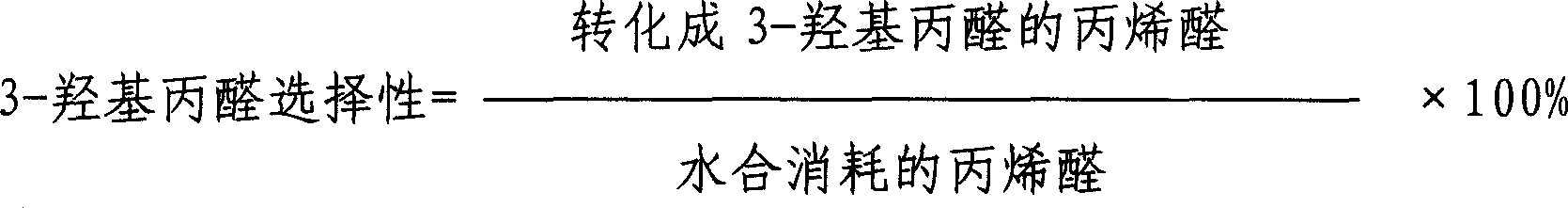
[0015] In a φ25mm×510mm stainless steel tubular reactor, 250ml of catalyst is loaded to form a fixed bed. The catalyst is a weakly acidic chelating cation exchange resin, and its surface anchors ammonium diacetate as the active center. The particle size of the catalyst is 0.40-1.25mm. The bulk specific gravity is 0.6-0.7g / ml. A thermal insulation jacket of circulating hot water is installed outside the reactor, and platinum resistors for temperature measurement are respectively installed on the upper, middle and lower parts of the catalyst bed of the reactor. The hydration raw material enters the catalyst bed after being preheated, the feeding amount of the hydration liquid is controlled by the feed pump, and the carbon dioxide pressure is regulated by the back pressure valve. The air in the reactor was replaced with nitrogen twice before the reaction. The concentration of the acrolein aqueous solution is 17.5wt%. The specific hydration reaction temperature, liquid hourly spa...
Embodiment 7
[0017] The catalyst is a weakly acidic chelating cation exchange resin, and its surface is anchored with thiodiacetamide as an active center, and the rest are the same as in Examples 1-6.
[0018] The specific hydration reaction temperature, liquid hourly space velocity, and partial pressure of carbon dioxide in the reaction system are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com