Muramidase detction reagent and its preparing method
A technology for detecting reagents and lysozyme, applied in the field of biochemical analysis, can solve the problems of increasing the difficulty of operation, difficult to adapt to the development trend of detection requirements, and the influence of subjective errors of test results operators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of lysozyme test reagent
[0021] 1. Use conventional culture methods to cultivate micrococcus on nutrient agar for 48 hours,
[0022] 2. Under aseptic conditions, wash the lawn with physiological saline,
[0023] 3. Use active chlorine to inactivate, the concentration is 1000ppm, the time is 30 minutes,
[0024] 4. Filter the inactivated micrococcus with gauze to remove the mixed agar fragments; wash the bacteria with normal saline, and then wash with 0.125mol / L pH6.0 phosphate buffer.
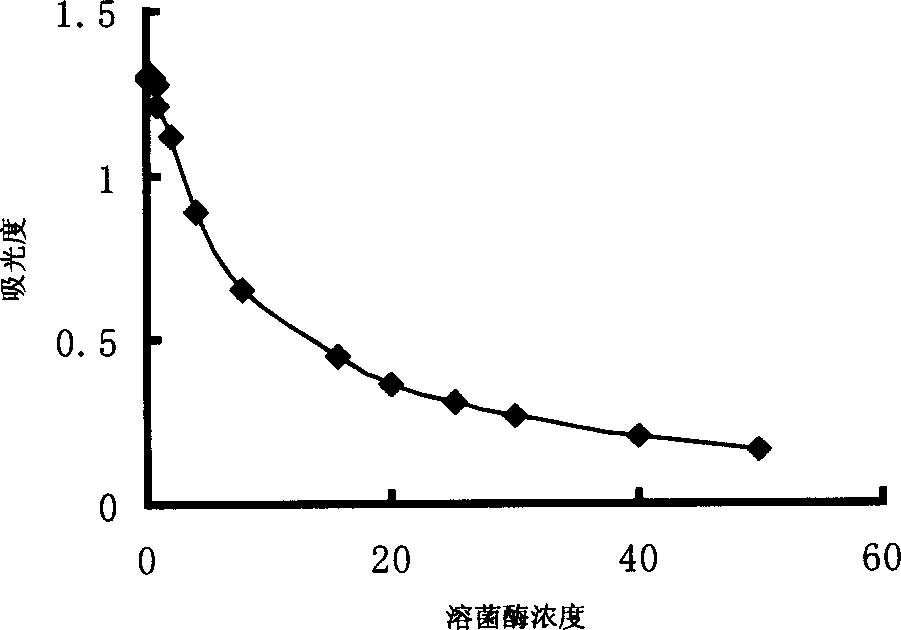
[0025] 5. Suspend with a phosphate buffer containing 1.3‰ gelatin and 1‰ sodium azide, and use the same buffer to adjust the bacterial suspension to a light path of 1cm and an absorbance of about 1.5A at a wavelength of 650nm to obtain a bacterial suspension. Store at 4°C to obtain the lysozyme test reagent of the present invention.
Embodiment 2
[0026] Example 2 Preparation of lysozyme test reagent
[0027] Use conventional culture methods to culture micrococcus on nutrient agar for 72 hours, wash the lawn with physiological saline under aseptic conditions, inactivate it with active chlorine at a concentration of 2000ppm for 15 minutes, filter the inactivated micrococcal gauze to remove the mixture Agar fragments; wash the bacteria with physiological saline, then wash with 0.0625mol / L pH6.0 phosphate buffer, suspend with 1.5‰ gelatin, 1‰ sodium azide in phosphate buffer, and use the same buffer Adjust the bacterial suspension to a light path of 1 cm and an absorbance of about 1.5 A at a wavelength of 650 nm to obtain the bacterial suspension. Store at 4°C to obtain the lysozyme test reagent of the present invention.
Embodiment 3
[0028] Example 3 Clinical test
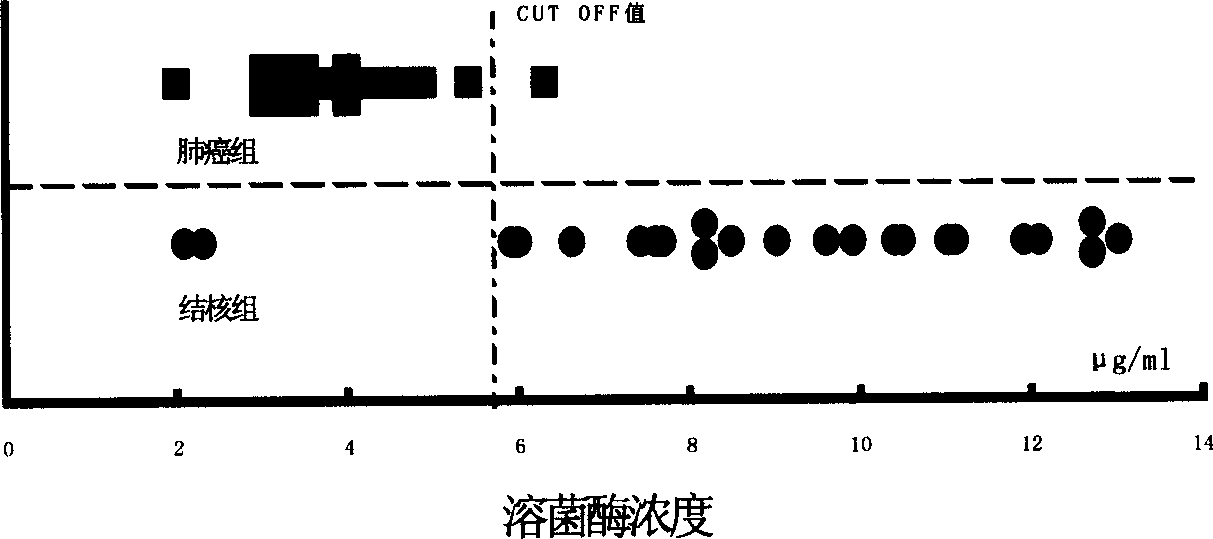
[0029] Fifty adult physical examination serum specimens were taken; 44 cases of pleural effusion specimens were taken. There were 21 cases of cancerous pleural effusion, all confirmed by cytology, 19 cases were lung adenocarcinoma, 1 case was suspected to be adenocarcinoma, 1 case was postoperative breast cancer; 23 cases were tuberculous pleural effusion. After the specimens are collected, they are frozen at -40°C. When testing, place at room temperature to order re-thawing, invert to mix, centrifuge and take the supernatant for inspection.
[0030] Use HITACHI 7060 automatic biochemical analyzer to establish an automatic analysis method for lysozyme activity. Method: endpoint method; wavelength: 340 / 623nm; reagent volume: 250μl; sample volume: 30μl; measurement points: 31 points. The lysozyme standard was prepared into a 15.6μg / ml solution as a calibration solution, and a 5-point calibration was performed.
[0031] Dilute the standard lysozyme us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com