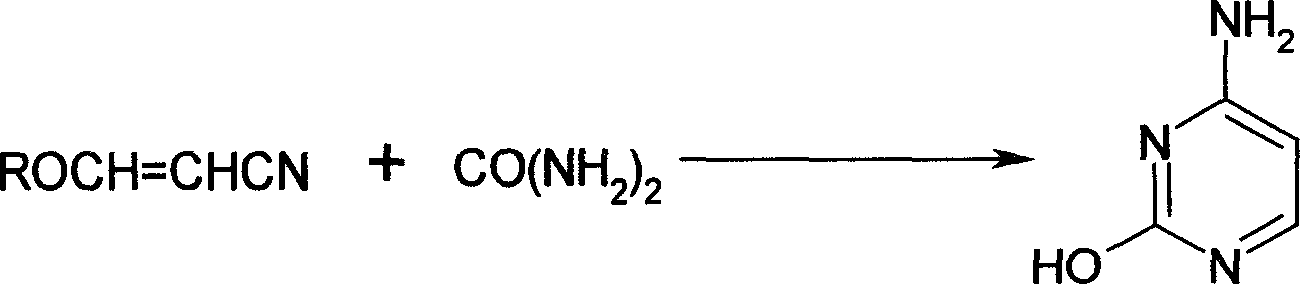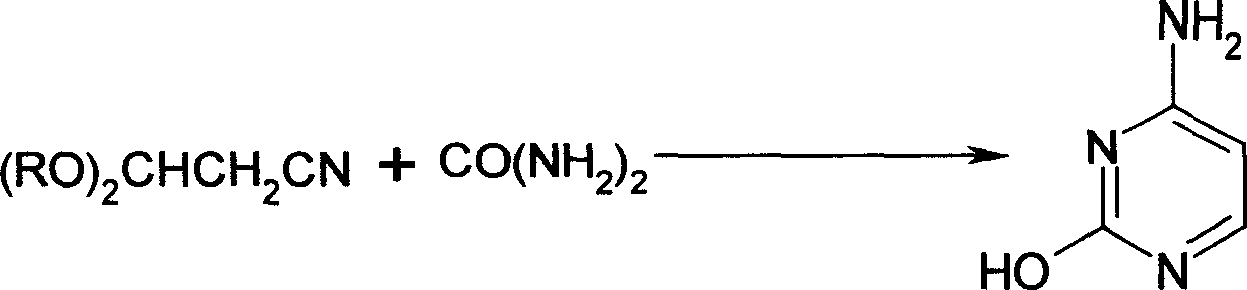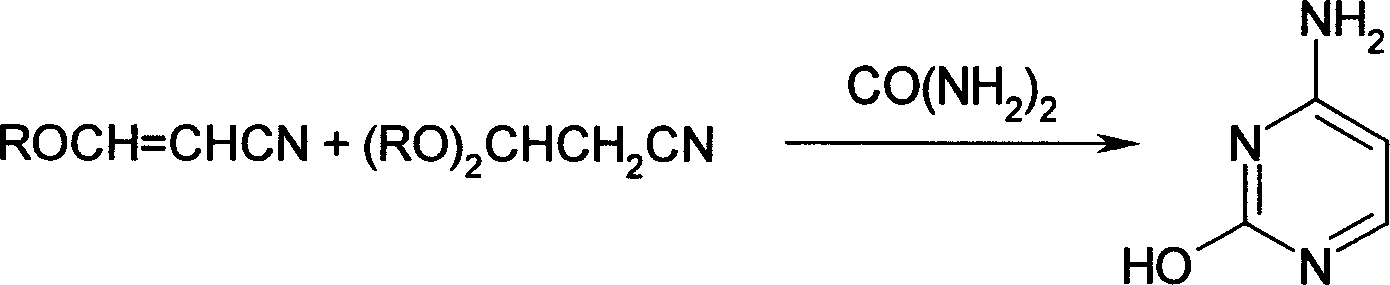Process for the preparation of 3-hydroxyacrylonitrile metal salts
A technology of hydroxyacrylonitrile and metal salts, applied in the direction of organic chemistry, etc., can solve the problems of high reaction operation pressure, complicated preparation method operation, and hidden safety hazards in operation, and achieve good yield, convenient preparation, and reduce unsafe factors Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 90 kg of acetonitrile, 400 kg of toluene, and 150 kg of sodium methoxide into a 1000-liter stainless steel reactor, and add 280 kg of methyl formate at 25°C. After the addition, stir for 5 hours under 3 atmospheres, that is The sodium salt solution of 3-hydroxyacrylonitrile was obtained.
[0038] Then, at 30°C, the sodium salt solution of the obtained 3-hydroxyacrylonitrile was added to a 2000-liter reaction kettle equipped with 700 kg of salt alcohol ethanol, and after stirring for 2.5 hours, it was neutralized to neutral with alkali, evaporated Remove the solvent, add 400 kg of toluene, 150 kg of sodium methoxide, and 200 kg of urea to the residue, react at 70°C for 4 hours, cool, neutralize with glacial acetic acid, filter, and recrystallize the obtained solid with acetic acid to obtain cytosine 130 kg, melting point: >280°C, content: 99.0% (HPLC, area normalization method), 99.8% (reference method). Based on acetonitrile, the yield is: 53.2%.
Embodiment 2
[0040] Add 90 kilograms of acetonitrile, 400 kilograms of toluene, and 150 kilograms of sodium methoxide in a 1000-liter stainless steel reactor, and add 300 kilograms of ethyl formate at 20 ° C. After the addition, continue stirring for 5 hours under 3 atmospheres, that is, The sodium salt solution of 3-hydroxyacrylonitrile was obtained.
[0041] Then, at 30°C, the sodium salt solution of the obtained 3-hydroxyacrylonitrile was added to a 2000-liter reaction kettle equipped with 700 kg of salt alcohol ethanol, stirred for 2.5 hours, neutralized with alkali to neutrality, and evaporated. Solvent, add 400 kg of toluene, 150 kg of sodium methoxide, and 200 kg of urea to the residue, react at 70°C for 4 hours, cool, neutralize with glacial acetic acid, filter, and recrystallize the obtained solid with acetic acid to obtain cytosine 137 kg, melting point: >280°C, content: 99.1%% (HPLC, area normalization method), 101.2% (HPLC, reference substance method). Based on acetonitrile, t...
Embodiment 3
[0043] Add 90 grams of acetonitrile, 400 grams of benzene, and 290 grams of sodium isopropoxide into a 1000 mL autoclave, and add 300 grams of isopropyl formate at 25°C. After the addition is complete, continue stirring for 5 hours at 3 atmospheres , That is, the sodium salt solution of 3-hydroxyacrylonitrile.
[0044] Then, at 30°C, the resulting sodium salt solution of 3-hydroxyacrylonitrile was added to a 2000 mL three-necked flask containing 700 grams of salt alcohol ethanol, stirred for 2.5 hours, neutralized to neutral with alkali, evaporated to Solvent, add 400 grams of xylene, 150 grams of sodium methoxide, and 200 grams of urea to the residue, react at 70°C for 4 hours, cool, add glacial acetic acid to neutralize, filter, and recrystallize the obtained solid with acetic acid to obtain cell 133 grams of pyrimidine, melting point: >280°C, content: 99.0%% (HPLC, area normalization method), 99.9% (HPLC, reference substance method). Based on acetonitrile, the yield is: 54...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com