Ornidazole effervescent tablet
A technology of ornidazole soaking and ornidazole, which is applied in the field of western medicine preparations, can solve the problems that affect the widespread use, aggravate the burden on the liver and kidney of patients, and kill pathogenic bacteria, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
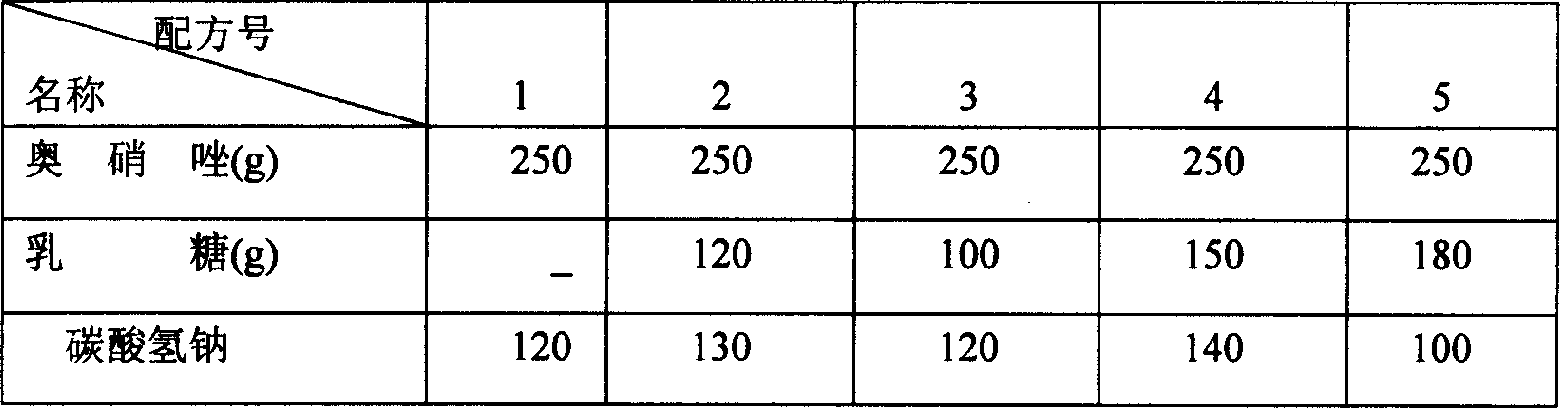
[0037] A. Based on 1000 tablets, 250 grams of ornidazole, 100 grams of lactose, 150 grams of tartaric acid, 90 grams of boric acid, 120 grams of sodium bicarbonate, 12 grams of Tween-80, 5 grams of povidone, low-substituted hydroxypropyl Base cellulose 20 grams, magnesium stearate 10 grams, sodium lauryl sulfate 10 grams, above-mentioned raw materials are respectively pulverized, cross 100 mesh sieves, 95% ethanol 100ml, set aside.
[0038] B dissolves Tween-80 and povidone in 95% ethanol to make adhesive, for subsequent use;
[0039] C. Make ornidazole, lactose, and sodium bicarbonate into a soft material with the binder in B above, granulate, and bake at 50-55°C.
[0040] D. Make tartaric acid and boric acid into soft materials with binders, granulate, and bake at 50-55°C.
[0041] F. The above-mentioned granules are sized respectively, mixed, added with low-substituted hydroxypropyl cellulose, sodium lauryl sulfate, and magnesium stearate, mixed evenly, and compressed into...
Embodiment 2
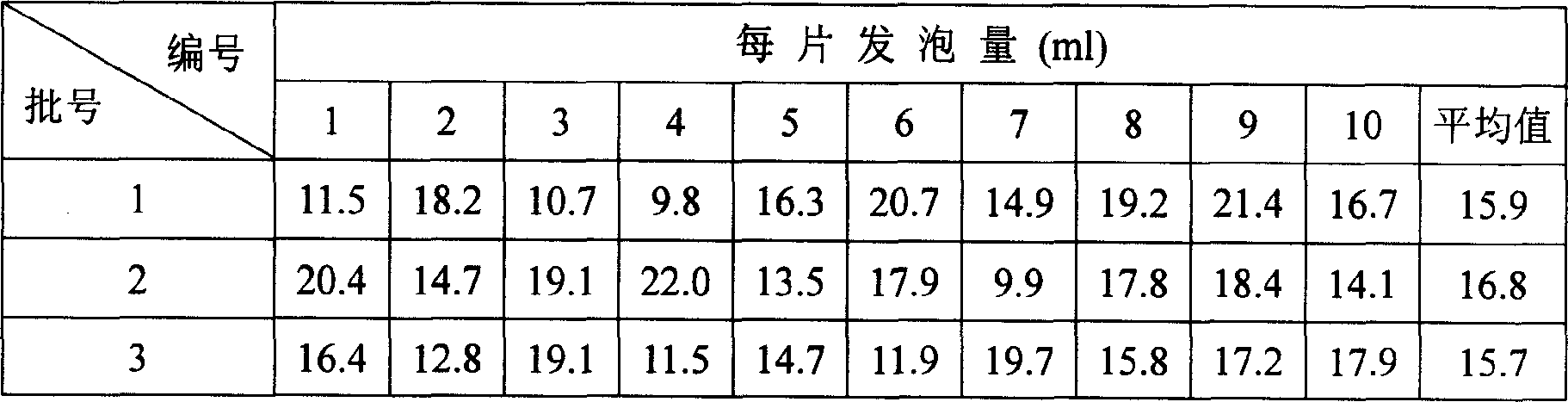
[0053] Vaginal Irritation Test of Ornidazole Effervescent Tablets
[0054] Test materials: drug: ornidazole effervescent tablet, specification: 250mg / tablet. Grind well during the test, and prepare the concentration to be 20mg / ml (referring to the content of ornidazole) with distilled water. The auxiliary material is prepared with distilled water so that the concentration is 40 mg / ml (referring to the auxiliary material content).
[0055] Animals: domestic rabbit, female, 2.0-2.5 kg, provided by Hunan Experimental Rabbit Supply Center.
[0056] experiment method
[0057] 12 rabbits were randomly divided into 3 groups, 4 in each group, (1) blank control group (distilled water), (2) excipient group (adjuvant 60mg / Kg). (3) Ornidazole effervescent tablet group (Ornidazole 30mg / Kg). Gently place the drug (1.5ml / Kg) in the vagina of the rabbit, administer it once a day, for 7 consecutive days, and ensure that the drug is in contact with the mucous membrane for at least 4 hours eve...
Embodiment 3
[0061] Acute Toxicity Test of Ornidazole Effervescent Tablets
[0062] experiment material
[0063] 1. Drug: Ornidazole effervescent tablet, specification: 250mg / tablet. During the test, take ornidazole and grind it evenly, and make it into a concentration of 300 mg / ml (referring to the content of ornidazole) with distilled water. The auxiliary material is prepared with distilled water to a concentration of 300 mg / ml (referring to the auxiliary material content).
[0064] 2. Animals: domestic rabbit, female, 2.0-2.5 kg, provided by Hunan Experimental Rabbit Supply Center.
[0065] experiment method
[0066] 12 rabbits were randomly divided into 3 groups, 4 in each group, (1) blank control group (distilled water), (2) excipient group (adjuvant 450 mg / Kg). (3) Ornidazole effervescent tablet group (Ornidazole 450mg / Kg). Gently place the drug (1.5ml / Kg) in the vagina of the rabbit to ensure that the drug is in contact with the mucous membrane for at least 4 hours. Immediately...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com