Production of mouse model of viral hepatitis B with high expression HBsAg
A viral hepatitis and mouse model technology, applied in preparations for in vivo experiments, pharmaceutical formulations, etc., can solve the problems of large differences in biological characteristics, cannot be directly applied to HBV infection, and cannot cause liver inflammation, etc., to achieve easy operation , The production process is simple and easy, and the production process takes a short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
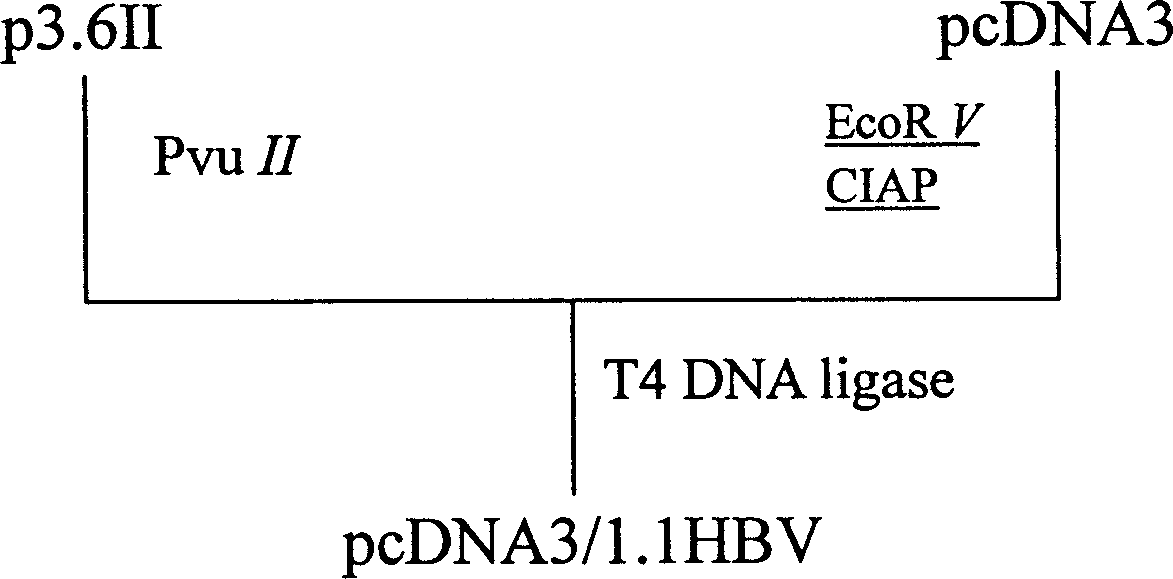
[0060] Embodiment 1: Construction of recombinant plasmid pcDNA3 / 1.1HBV
[0061] 1. Materials
[0062] The plasmid p3.6II containing 1.1 times the full gene of hepatitis B virus was kindly donated by Professor Wang Yuan from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Plasmid extraction kits and DNA recovery kits are Qiagen products. The BioPhotometer quantitative instrument is a product of Eppendorf in Germany, the automatic gel imaging system is a product of AmershamPharmacia Biotech in the United States, the ZF-BI ultraviolet spectrophotometer is manufactured by Shanghai Changming Electronic Instrument Factory, and the HZS-H constant temperature water bath oscillator is manufactured by Harbin East The product of Lian Electronic Technology Development Co., Ltd., the digital display electric heating constant temperature water bath box (HH·W21·600S) is manufactured by Shanghai Yuejin Medical Equipment Factory, and the LKB2103 electrophores...
Embodiment 2
[0073] Embodiment 2: Amplification, extraction and purification of recombinant plasmid pcDNA3 / 1.1HBV
[0074] 1. Materials
[0075] (1) Plasmid: The recombinant plasmid pcDNA3 / 1.1HBV carrying 1.1 copies of the whole HBV genome was constructed and preserved by our laboratory. The recombinant plasmid pcDNA3 / 1.1HBV was stored in Escherichia coli DH5α.
[0076] (2) Reagents: The plasmid mass extraction kit is a product of QIAGEN, Germany. The kit contains 10 pieces of QIAGEN-tip500, Buffer P1 1×110ml, Buffer P2 1×110ml, Buffer P3 1×110ml, Buffer QBT 1×110ml, Buffer QC 3×240ml, Buffer QF 1×170ml, RNase A*1× 11 mg.
[0077] (3) Instruments: The gel automatic imaging system is a product of Amersham Pharmacia Biotech in the United States, the ZF-BI ultraviolet spectrophotometer is manufactured by Shanghai Changming Electronic Instrument Factory, and the HZS-H constant temperature water bath oscillator is a product of Harbin Donglian Electronic Technology The product of Development...
Embodiment 3
[0101] Example 3: Tail vein high pressure injection to establish mouse hepatitis model
[0102] 1. Materials
[0103] (1) Animals: SPF-grade BALB / c mice, 4-6 weeks old, male, weighing 18-22 g, provided by the Experimental Animal Center of Shandong University, and the feeding conditions were in accordance with the standards for SPF-grade animals.
[0104] (2) Reagent: pcDNA3-HBV1.1 purified by QIAGEN Plasmid Mass Extraction Kit, adjusted concentration to 20-50 μg / ml; physiological saline (0.9% NaCl solution).
[0105] (3) Instruments: 1ml and 5ml syringes; 0.55mm needle.
[0106] 2. Method
[0107] (1) Tail vein high-pressure injection: first place the mouse in a high-temperature environment to expand the tail vein; anesthetize the mouse with ether before injection, and observe the reflex of the mouse; within 5 seconds, inject 1.4-2.0ml of liquid into the small vein through the tail vein at a uniform speed. In mice, the mice were placed at 25-28°C.
[0108] (2) Blood collec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com