Method for defluorinating and degrading complete fluorine substituted compounds
A compound and defluorination technology, applied in the field of decomposition of perfluorinated substituted organics, can solve problems such as ineffective decomposition of PFOA and PFOS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the vacuum ultraviolet ray reduction decomposition of perfluorooctanoic acid (PFOA)
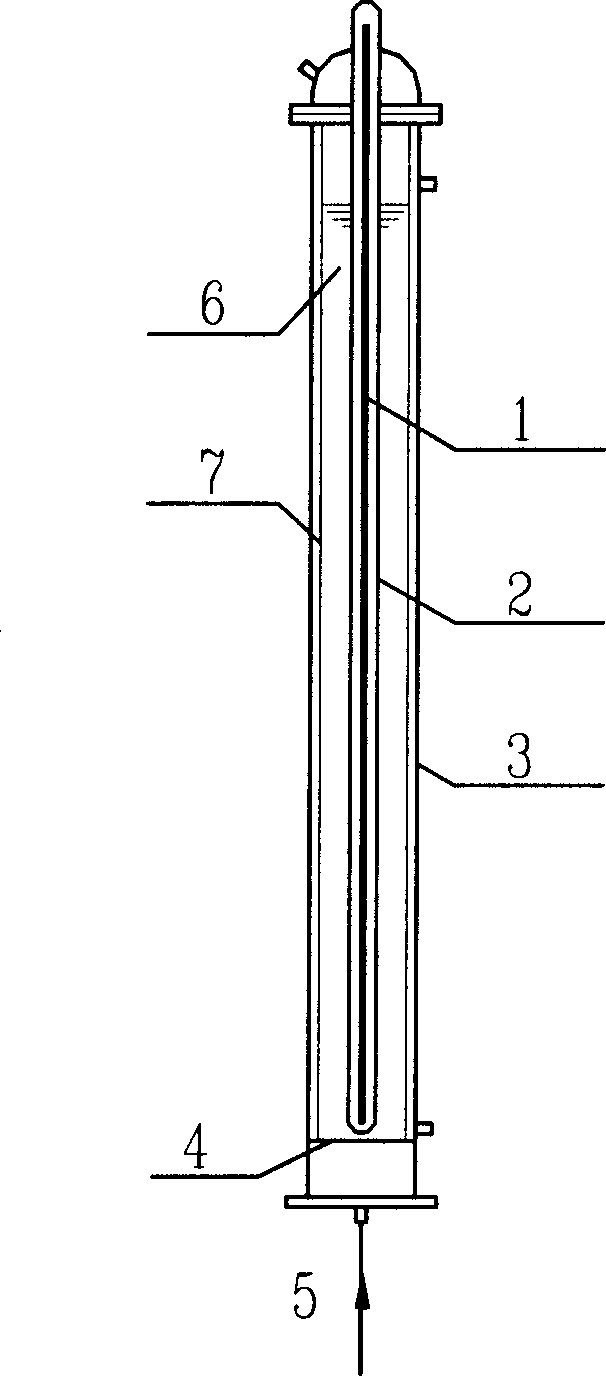
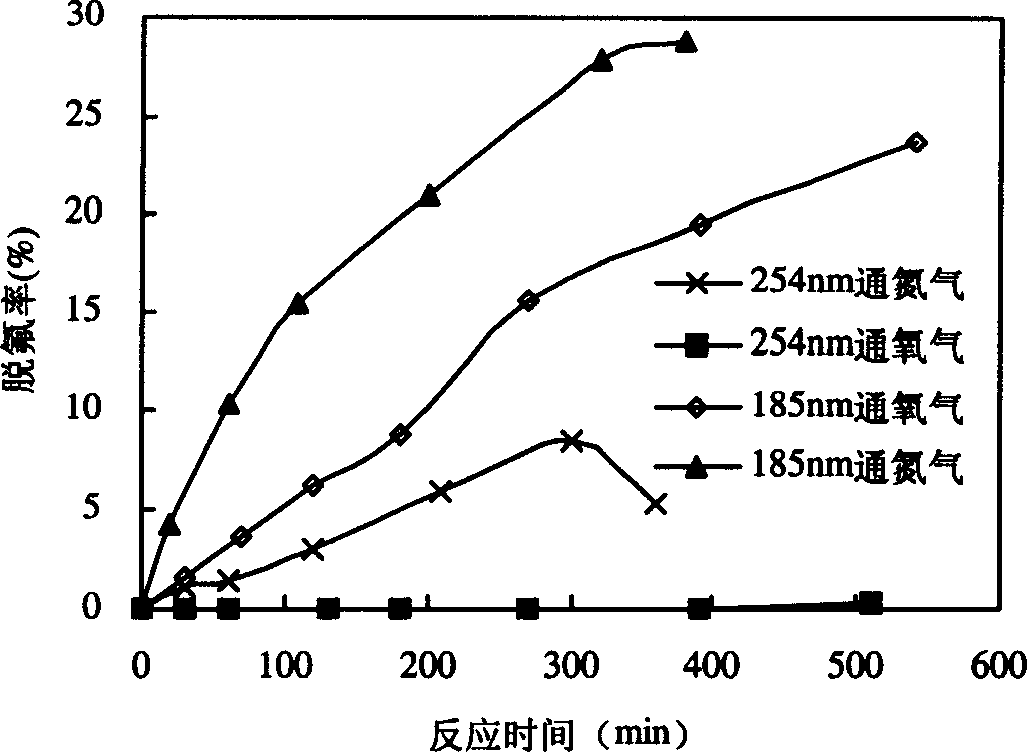
[0026] Such as figure 1 As shown, 800mL of PFOA aqueous solution containing 15mg / L is put into reactor 3, nitrogen gas is passed into reactor 3 from bottom gas distribution plate 4, and under the irradiation of 15W emitting 185nm vacuum ultraviolet ray lamp 1, the defluorination and decomposition of PFOA See the effect figure 2. The defluorination rate is calculated according to the fluoride ion concentration in the water during the reaction, and the fluoride ion concentration in the water is measured by fluorine reagent spectrophotometry. Nitrogen gas under 185nm vacuum ultraviolet irradiation ( figure 2 marked as "185nm nitrogen flow"), the concentration of fluoride ions in the reaction solution increased with the reaction time, and the defluorination rate of PFOA reached 28.7% after 380 minutes of reaction, and 15 fluorine (F) 4.3 fluorines had fallen into soluti...
Embodiment 2
[0027] Embodiment 2: the vacuum ultraviolet ray reduction decomposition of perfluorooctane sulfonate (PFOS)
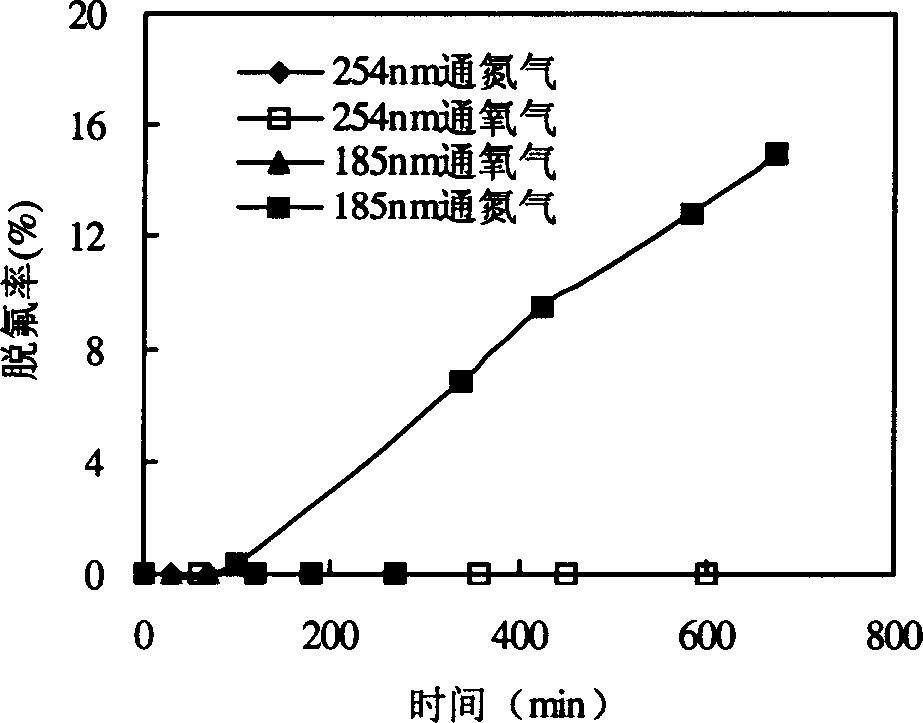
[0028] Such as figure 1 As shown, 800mL of PFOS aqueous solution containing 20mg / L is put into reactor 3, nitrogen gas is passed into reactor 3 from bottom gas distribution plate 4, and under the irradiation of 15W emitting 185nm vacuum ultraviolet ray lamp 1, the effect of defluorination and decomposition of PFOS See image 3 , using fluorine reagent spectrophotometry to measure the fluoride ion concentration in the solution. The fluoride ion concentration in the reaction solution increases with the reaction time. After 675 minutes of reaction, the defluorination rate of PFOS reaches 15%. On average, 17 ions contained in PFOS molecules Fluorine (F) has 2.55 fluorine dropped into the solution. In the case of 185nm vacuum ultraviolet radiation and oxygen, PFOS does not defluorinate and decompose; under 254nm radiation, no matter whether oxygen or nitrogen is introduce...
Embodiment 3
[0029] Example 3: Catalytic defluorination and decomposition of perfluorooctanoic acid by vacuum ultraviolet light
[0030] Such as figure 1 As shown, 800mL of PFOA aqueous solution containing 19mg / L is put into reactor 3, nitrogen is passed into reactor 3 from the bottom gas distribution plate, and semiconductor photocatalyst LiNbO is added at the same time 3 , the concentration in the reaction solution is 0.1g / L, and under the irradiation of 15W emitting 185nm vacuum ultraviolet ray lamp 1, the effect of PFOA defluorination and decomposition is shown in Figure 4 . Measure the concentration of fluoride ion in the reaction solution with fluorine reagent spectrophotometry, the concentration of fluoride ion in the reaction solution increases with the reaction time, the defluorination rate of PFOA reaches 23.5% after reacting for 210 minutes, on average, 15% contained in the PFOA molecule A fluorine (F) has 3.5 fluorine dropped into the solution. And correspondingly, if LiNbO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com