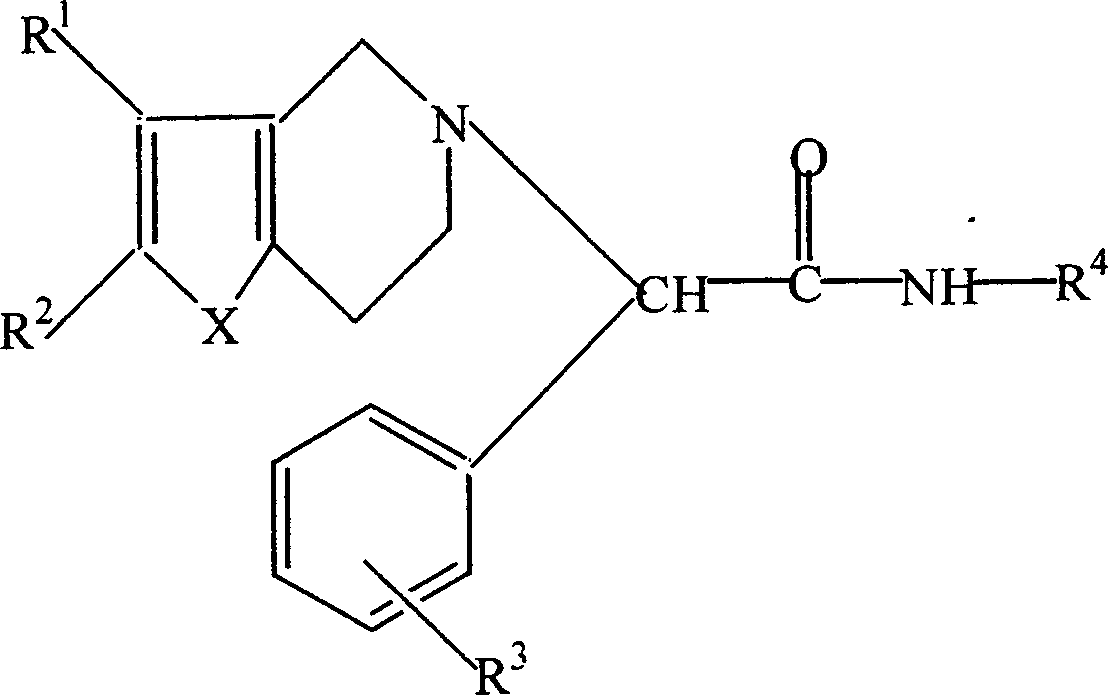
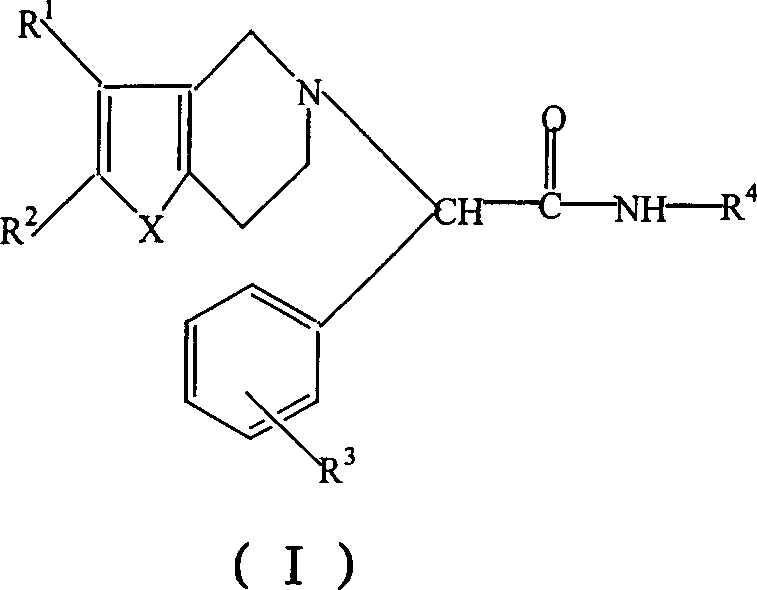
Thiophenopyridine substituted acetyl hyarazine derivative
A technology of pyridine and tetrahydrothiophene, applied in the field of medicine, can solve problems such as weak alkalinity and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0079] (S)-α,α-[(2-chlorophenyl)-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)]acetylhydrazide (compound 1) (hereinafter referred to as hydrazide)
[0080] In the reaction bottle equipped with stirring, condenser and thermometer, add 38g of clopidogrel and 30ml of absolute ethanol, heat slowly under stirring to dissolve the reaction raw materials, add 45.6g of hydrazine hydrate (80%), and continue heating to reflux , insulation reaction for 4 hours (the plate layer shows that the reaction is complete). Then the solvent was evaporated under reduced pressure, and after evaporation, 50ml of distilled water and 30ml of dichloromethane were added to the residue, fully stirred, and the organic layer was separated. Sodium is fully dry. Dichloromethane was evaporated under reduced pressure to obtain 23.4 g of white solid. (HPLC: 97.16%), m.p.139.0°C-139.3°C.
Embodiment 2
[0082] (S)-α,α-[(2-chlorophenyl)-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)]-N'-[( E)-(Styryl)methylidene]acetylhydrazide (Compound 2)
[0083] Add 4 g of hydrazide and 40 ml of anhydrous methanol into a reaction flask equipped with stirring, a condenser, and a thermometer, start stirring, and heat to dissolve. Continue heating to 40°C, add 1.8g cinnamic aldehyde dropwise, after the addition is complete, keep the reaction for 3 hours. The reaction was stopped and cooled to produce a yellow solid. Filter, wash with anhydrous methanol 3×2ml, and dry to obtain 4.4g of yellow solid. (HPLC: 97.92%), m.p.173.3°C~174.2°C, Rf=0.65 (developing solvent: petroleum ether (60°C~90°C):ethyl acetate=1:1)
Embodiment 3
[0085] (S)-α,α-[(2-chlorophenyl)-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)]-N'-[( E)-(4-methoxyphenyl)methylidene]acetylhydrazide (compound 3)
[0086] The compound is obtained by the reaction of hydrazide and p-methoxybenzaldehyde according to the preparation process provided in Example 2.
[0087] HPLC: 95.92%, m.p.193.3-194.6°C, Rf=0.54 (developing solvent: petroleum ether (60-90°C):ethyl acetate=1:1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com