Method for analyzing and separating preparation of Huperzine A and Huperzine B
A technology of Huperzine A and Huperzine B, applied in the field of analytical chemistry, can solve the problems of low yield, discard, long process cycle and the like, and achieve the effects of low separation, good presentation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
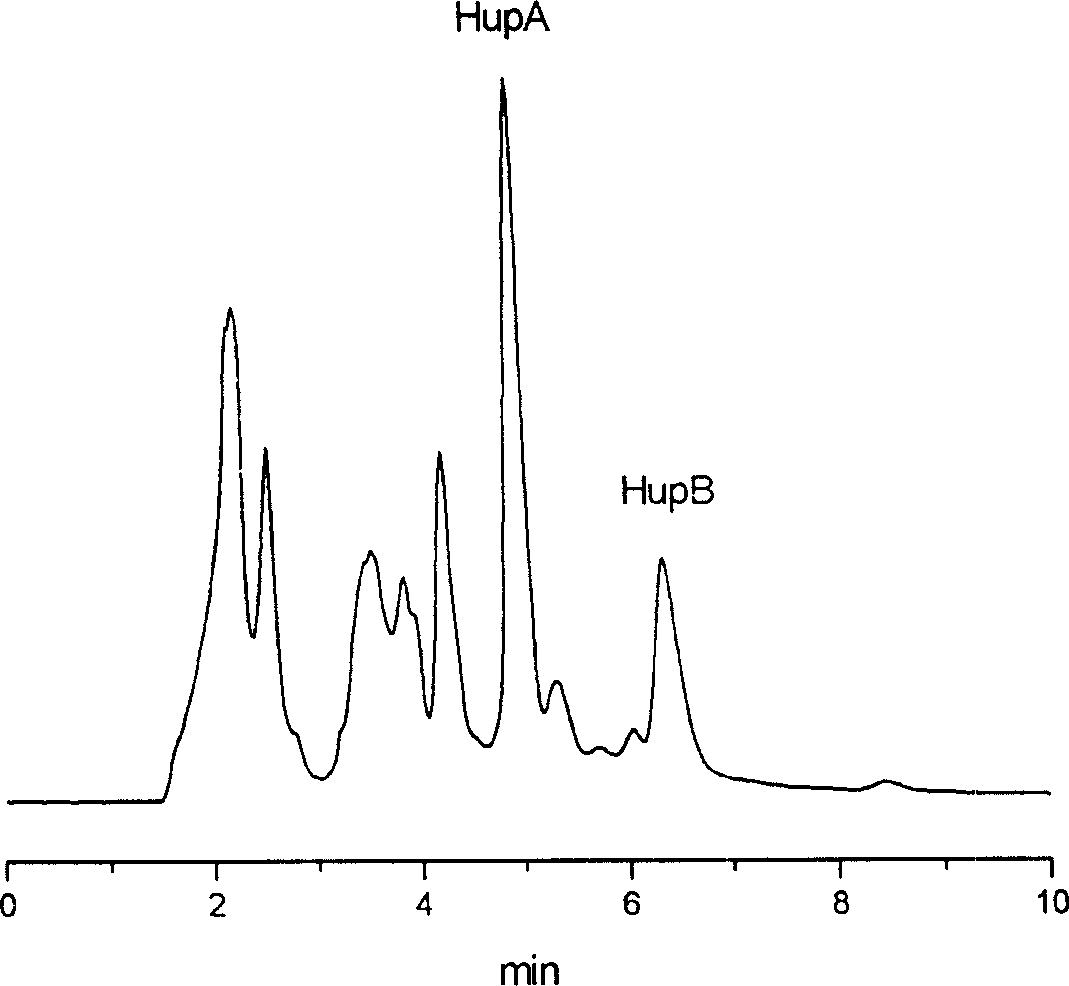
[0032]Embodiment 1, with the huperzine A 0.053% containing, the huperzine B 0.013% phyllocolumn as raw material, separation and purification preparation 99.1% huperzine A and 98.4% huperzine B, the process adopts high-efficiency liquid phase The content of huperzine A and huperzine B was analyzed by chromatography.
[0033] The HPLC analysis conditions of the sample are: Waters Alliance 2695 high performance liquid chromatograph, 2996 ultraviolet diode array detector, chromatographic column filler is phenyl bonded phase silica gel Inertsil Ph-3, mobile phase methanol: water=90: 10, sample injection The volume is 20 μl, and the flow rate is 1ml / min.
[0034] 1) After 1 kg of dry Melaleuca tower containing huperzine A 0.053% and huperzine B 0.013% is pulverized, soak for 24 hours with 10 liters of 0.1% nitric acid; after pouring out the supernatant, add 5 liters to the residue Soak in 0.1% nitric acid for 12 hours; filter and wash the filter residue several times with the same ...
Embodiment 2
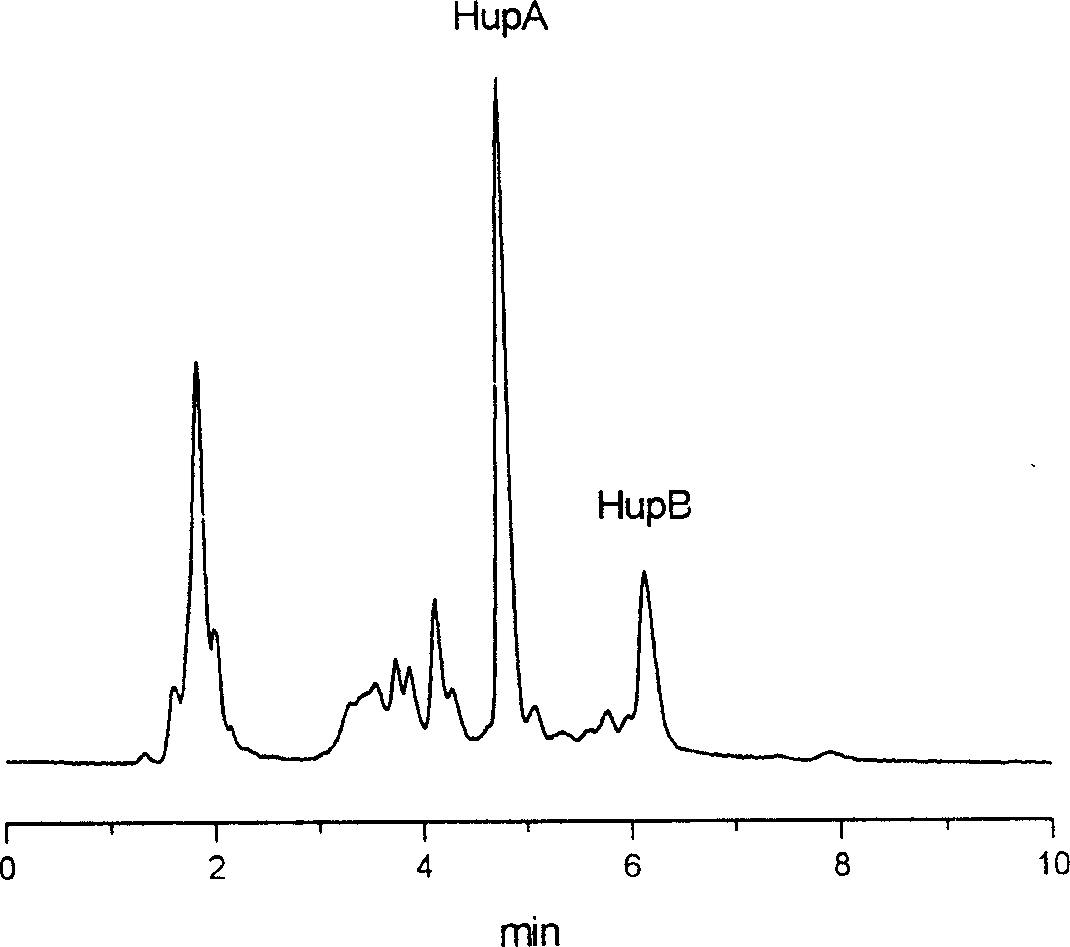
[0040] Embodiment 2, with the huperzine A 0.016% containing, the huperzine B 0.0033% melaleuca tower as raw material, separation and purification prepare 98.5% huperzine A and 98.2% huperzine B, the process adopts high-efficiency liquid phase The content of huperzine A and huperzine B was analyzed by chromatography.
[0041] The HPLC analysis method of sample is: Beckman FL750 high performance liquid chromatograph, ultraviolet multi-wavelength detector, chromatographic column filler is pentafluorophenyl bonded phase silica gel Curosil-PFP, mobile phase methanol: water=70: 30, sample volume 10μl, flow rate 2ml / min.
[0042] 1) After pulverizing the dried Melaleuca tower containing 0.016% of huperzine A and 0.0033% of huperzine B, soak it with 1% sulfuric acid for 24 hours; after pouring out the supernatant, add 1% sulfuric acid to the residue to soak for 12 hours; filter, wash the filter residue several times with the same acid solution; combine the filtrate and washing liquid...
Embodiment 3
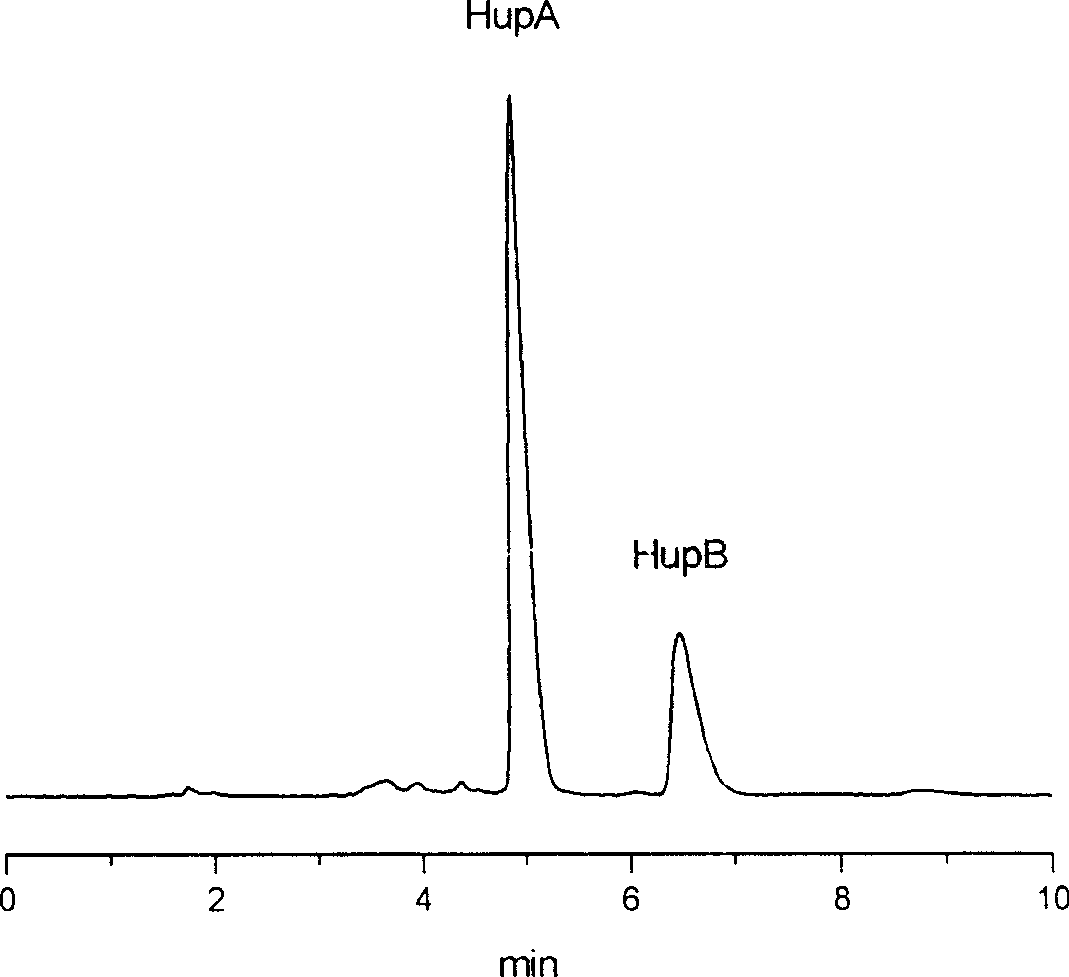
[0048] Embodiment 3, with the huperzine A 0.0035% containing, the huperzine B 0.0014% melaleuca tower as raw material, separate and purify and prepare 98.5% huperzine A and 98.1% huperzine B, the process adopts high-efficiency liquid phase The content of huperzine A and huperzine B was analyzed by chromatography.
[0049] The HPLC analysis method of sample is: Agilent 1100 high-performance liquid chromatograph, ultraviolet multi-wavelength detector, chromatographic column filler is nitrile-based bonded phase silica gel Lichrospher 100 CN, mobile phase methanol: water=60: 40, and the injection volume is 20 μ l , flow rate 0.5ml / min.
[0050] 1) After pulverizing the dried Melaleuca tower containing 0.053% of huperzine A and 0.013% of huperzine B, soak it with 2% sulfuric acid for 36 hours; after pouring out the supernatant, add 2% sulfuric acid to the residue and soak it for 12 hours; filter, wash the filter residue several times with the same acid solution; combine the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com