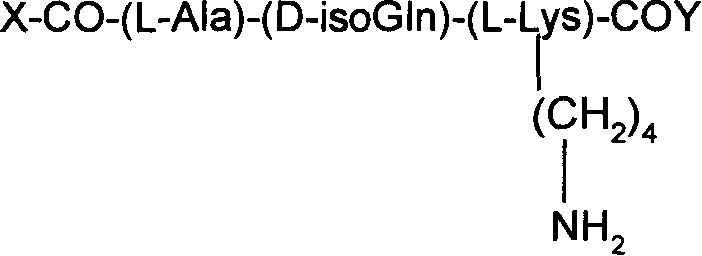Production and use for taxol and muramic acyl dipeptide conjugate substance of immune reinforcer
A technology of muramyl dipeptide and paclitaxel derivatives, which is applied in medical preparations of non-active ingredients, organic chemistry, and pharmaceutical formulations, and can solve problems such as fatalities, low water solubility of paclitaxel, and allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
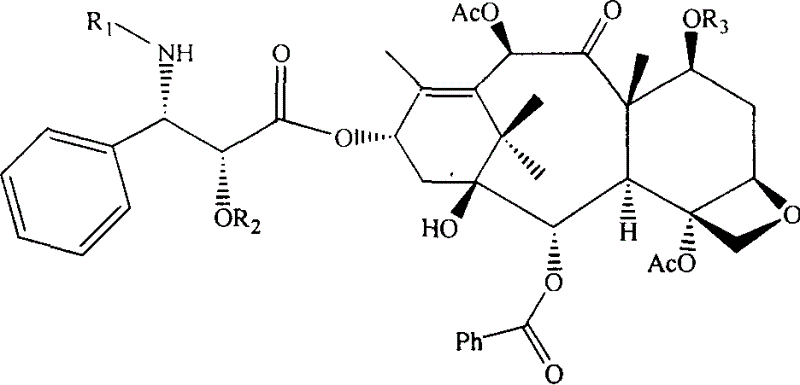
[0038] Example 1: Synthesis of 7-silyletherified-10-deacetyl-Baccatin III:
[0039] Dissolve 1.1g (2.0mmol) of 10-deacetyl-Baccatin III in 20ml of anhydrous pyridine, blow in nitrogen gas, slowly add 3.4ml (20mmol) of triethylsilyl chloride dropwise under stirring at room temperature, react for 10 hours, and add an appropriate amount of acetic acid Ethyl ether was diluted, and the dilutions were sequentially washed with saturated CuSO 4 solution (200ml×3) and water, and the organic layer was washed with anhydrous Na 2 SO 4 Dried and concentrated to obtain 1.1 g of white powder with a yield of 84%.
Embodiment 2
[0040] Embodiment 2: Synthesis of 7 silicon etherification-Baccatin III:
[0041] Dissolve 0.5g (0.76mmol) of 7-silicate-10-deacetyl-Baccatin III in 30ml of anhydrous pyridine, blow in nitrogen, add 1.5ml of acetic anhydride (15.4mmol) under ice-cooling, and place at room temperature Stir for 72 hours, add an appropriate amount of ethyl acetate to dilute, and then wash with saturated CuSO 4 solution (200ml×3) and water, and the organic layer was washed with anhydrous Na 2 SO 4 After drying and concentration, 335 mg of white powder was obtained, with a yield of 63%.
Embodiment 3
[0042] Example 3: Synthesis of 7-acetoxy-Baccatin III:
[0043] Dissolve 2.0 g (3.676 mmol) of 10-deacetyl-Baccatin III in 100 ml of anhydrous pyridine, add 15 ml of acetic anhydride at room temperature, and stir at room temperature for 3 days. Concentrate under reduced pressure to remove excess pyridine, and recrystallize with methanol / water to obtain 2.31 g of white needle crystals (containing 19% of 7,10,13-triacetoxy Baccatin III), with a yield of 81.0%, which can be used for protection without recrystallization. Synthesis of Taxol. Purify by column chromatography to obtain pure 7-acetoxy-Baccatin III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com