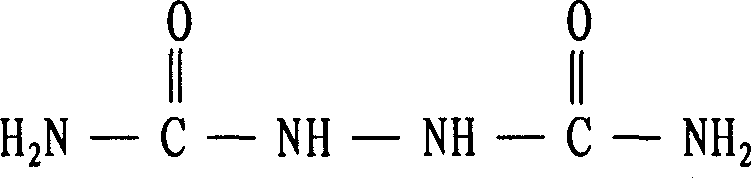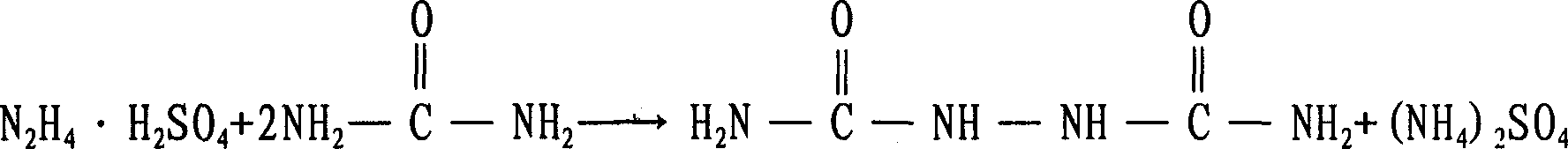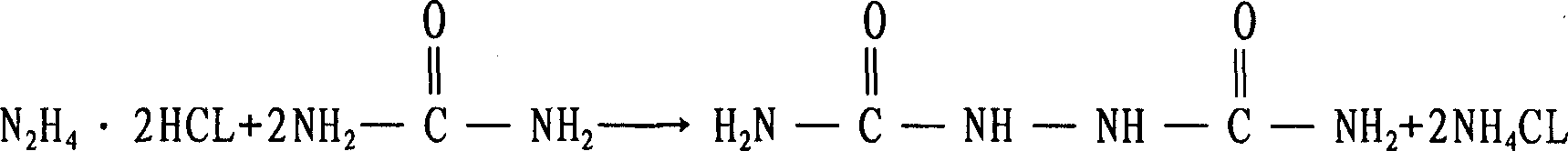Biurea synthesis technology
A synthesis process, biurea technology, applied in the field of biurea synthesis process, can solve the problems of high treatment costs, unaffordable enterprises, large water consumption, etc., to reduce production costs, ensure non-polluting discharge, and thoroughly treat waste liquid Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The following production steps are used:
[0021] 1. Pretreatment of HCL gas
[0022] Pass HCL gas into it and fill it with FeCl as a reducing agent 2 Pretreatment is carried out in the filter tank to eliminate the excess oxidizing substances present.
[0023] 2. Synthesis of biurea
[0024] Add 500ml of hydrazine hydrate (hydrazine content 30g / L) into a 1000ml four-neck round-bottomed flask equipped with a mechanical stirrer, a thermometer, a reflux condenser and an air guide tube. 57g, and then feed the pretreated HCL gas through the airway until the pH value is 4, then warm to 108°C, adjust the amount of HCL gas feeding, and keep the pH value and reaction temperature to the end of the reaction (ie: in the solution hydrazine content <0.1g / L), and finally through washing, filtering and drying, 51.93g of biurea with a purity of 98% can be obtained, and the calculated reaction yield (in terms of hydrazine hydrate) is 92%.
[0025] The reaction formula in the above-me...
Embodiment 2
[0036] The following production steps are adopted:
[0037] 1. Pretreatment of hydrochloric acid
[0038] Pass HCL gas into it and fill it with FeSO as reducing agent 4 Pretreatment is carried out in the filter tank to eliminate the excess oxidizing substances, and then, the pretreated HCL gas is absorbed by water to make hydrochloric acid.
[0039] 2. Synthesis of biurea
[0040] Add 500ml of hydrazine hydrate (hydrazine content 29g / L) into a 1000ml four-necked round-bottomed flask with a mechanical stirrer, a thermometer, a reflux condenser and a constant pressure dropping funnel on it. Add 55.825g of urea in the ratio of 55.825g, and then add dropwise the pretreated hydrochloric acid (31%-36%) to the pH value of 5 through the constant pressure dropping funnel, and then heat up to 108°C to adjust the dropwise addition of hydrochloric acid. Quantity and rate of addition, keep above-mentioned pH value and temperature of reaction to the end of reaction, finally, through wash...
Embodiment 3
[0046]The following production steps are adopted:
[0047] 1. Carry out pretreatment respectively to HCL gas and hydrochloric acid, its processing method is identical with the processing method in embodiment 1, embodiment 2, but the reducing agent that adopts is Na 2 SO 3 .
[0048] 2. Synthesis of biurea
[0049] Add 500ml of hydrazine hydrate into a four-necked round-bottomed flask with a mechanical stirrer, a thermometer, a reflux condenser, and an airway tube of 1000ml. HCL gas, until the pH value is 5, replace the gas guide tube with a constant pressure dropping funnel that drips hydrochloric acid inward, raise the temperature to 107°C, adjust the amount and rate of hydrochloric acid added dropwise, and maintain the above pH value and reaction temperature to the end of the reaction (hydrazine content<0.1g / L), after washing, filtering and drying, 51.63g of biurea with a purity of 97.5% was obtained, and the calculated yield was 91% (in terms of hydrazine hydrate).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com