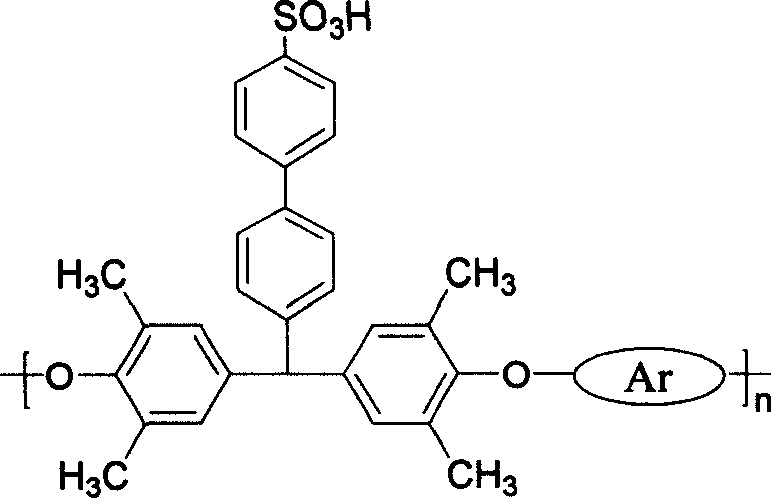
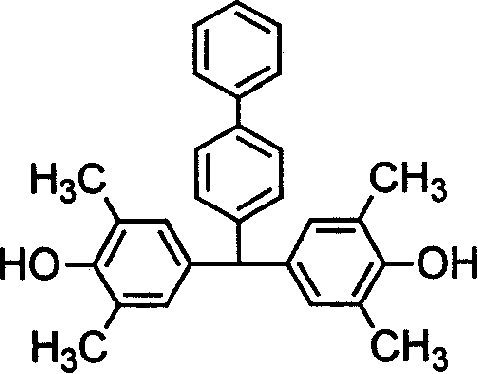
Sulfomated polyarylether containing conjugation structure, preparation method and intermediate
A technology of monomer and meaning, applied in the field of polyarylene ether, can solve the problems affecting the service life of PEMFC, instability of proton exchange membrane, easy cracking, etc., and achieve the effect of ensuring chemical stability, high strength and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of above formula (3) bisphenol monomer
[0034] Add 10.92g (0.06mol) of biphenyl formaldehyde, 21.96g (0.18mol) of 2,6-dimethylphenol and 30mL of toluene into a 100mL three-necked flask, stir to dissolve it, and add it dropwise at room temperature 10 mL of 60% (0.094 mol) sulfuric acid. N 2 Under protection, heat up to 50-55°C and stir for 20 hours, cool to room temperature, a red solid precipitates out, filter, wash twice with a small amount of water, once with a small amount of toluene, and dry in vacuo. Recrystallized twice with methanol-water at a volume ratio of 3:1 to obtain a white solid with a yield of 63%, which was confirmed to be the bisphenol monomer of the above formula (3) by analysis. The analysis data are as follows: M.p: 165~166℃; 1 H-NMR (CDCl 3 ): δ (ppm) 2.18 (s, 12H), 5.31 (s, 1H, CH), 6.73 (s, 4H, 2C 6 h2 ), 7.14~7.58 (m, 9H, C 6 h 5 C 6 h 4 ); MALDI-TOF-MS: found 408.1; calcd for C 29 h 28 o 2 , 408.5; ELE...
Embodiment 2
[0036] With the bisphenol monomer of 1mmol (0.408g) above-mentioned formula (3), in 1mmol (0.254g) above-mentioned formula (4) A difluoro monomer (purchased from Aldrich), 1.5mmol (0.208g) of anhydrous potassium carbonate, 5mL of toluene and 2.0mL of DMAC were added to a three-necked flask, under the protection of nitrogen, the temperature was raised to 140°C, and kept under magnetic stirring For 4 hours, distill out the toluene and take out the produced water, then raise the temperature to 170°C, react for 4 hours, dilute with DMAC, drop it into methanol with a small amount of HCI, filter, dry, dissolve with chloroform, and place for 6 hours Hour, filter, concentrate, drop in methanol, filter, dry, obtain white flocculent solid, productive rate 90.5%, prove to be the compound of formula (2) by analysis, wherein Be a, n=165, Waters510 HPLC measures relative molecular mass: M n b = 43 383, M w b =102 790, its glass transition temperature T g =261.4°C, the temperature T a...
Embodiment 3
[0041] With the bisphenol monomer of 1mmol (0.408g) above-mentioned formula (3), in 1mmol (0.322g) above-mentioned formula (4) The difluoromonomer b (purchased from Aldrich), 1.5mmol (0.208g) of anhydrous potassium carbonate, 5mL of toluene and 2.4mL of DMAC were added to a three-necked flask, under the protection of nitrogen, the temperature was raised to 135°C, and kept under magnetic stirring. For 4 hours, distill out the toluene and take out the water produced, then raise the temperature to 180°C, react for 3 hours, dilute with DMAC, drop it into methanol with a small amount of HCl, filter, dry, dissolve with chloroform, and place for 4 hours Hour, filter, concentrate, drop in methanol, filter, dry, obtain white flocculent solid, productive rate 92.4%, prove to be the compound of formula (2) by analysis, wherein For b, n=172, Waters 510 HPLC measures the relative molecular mass: M n b =58 484, M w b =118 820, glass transition temperature T g =248.9°C, the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com