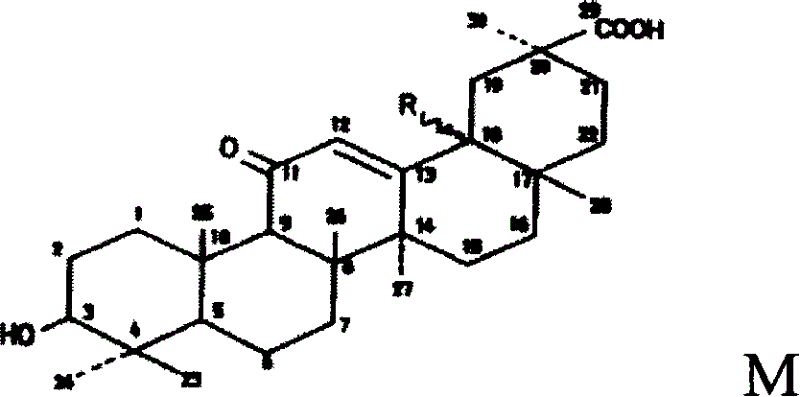
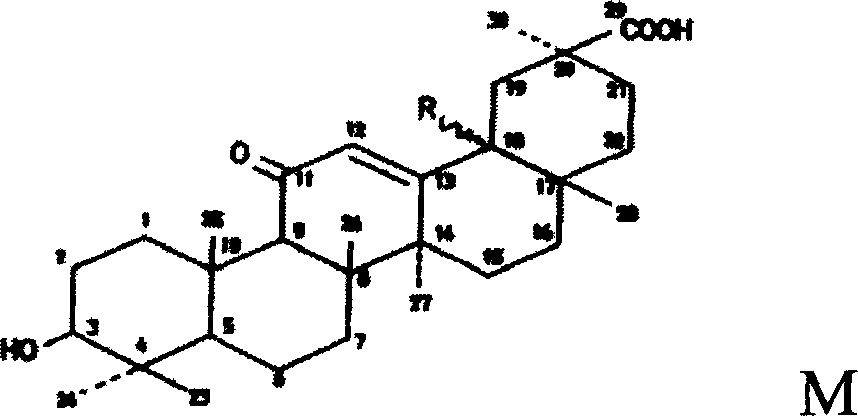
Enoxolone derivative, preparation method and uses
A technology of glycyrrhetinic acid and glycyrrhetinic acid salt, which is applied in the direction of medical formula, drug combination, drug delivery, etc., can solve the problems of limiting the clinical application of glycyrrhetinic acid, the inability to prepare injections, and vascular irritation, so as to reduce irritation and Effects of vasospasm, resolution of solubility, and improvement of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of glycyrrhetinic acid arginine
[0032] Add glycyrrhetinic acid 2.35g (0.005mol), L-arginine 0.87g (0.005mol), 90% ethanol (V / V) 440mL successively in the reaction flask, stir and react for 2 hours, evaporate the solvent under reduced pressure, and Add 20 mL of ethyl acetate to the residue, grind to obtain a light yellow precipitate, filter, and dry under reduced pressure to obtain 3.0 g of a light yellow solid with a yield of 93%. Glycyrrhetinic acid was used as the reference substance for content determination, and the product content was 99.7%.
[0033] Arginine Glycyrrhetinate exhibits the following physicochemical properties:
[0034] Properties: light yellow powder mp: 183~186℃.
[0035] ESI-MS m / z: 644 ([M-H] + ).
[0036] Elemental analysis % calculated value (found value): C 67.05 (67.48), H9.38 (9.27), N8.69 (8.81).
[0037] Molecular formula: (C 36 h 60 N 4 o 4 )
[0038] UV lambda max nm: 258
[0039] IR(KBr, v max...
Embodiment 2
[0041] Embodiment 2: the preparation of glycyrrhetinic acid arginine
[0042] Add glycyrrhetinic acid 2.35g (0.005mol), L-arginine 0.87g (0.005mol), 85% ethanol (V / V) 440mL successively in reaction bottle, stir reaction 2 hours, evaporate to dryness under reduced pressure, in Add 20 mL of ethyl acetate to the residue, grind to obtain a light yellow precipitate, filter, and dry under reduced pressure to obtain 3.1 g of a light yellow solid with a yield of 96%. Glycyrrhetinic acid was used as the reference substance for content determination, and the product content was 99.6%.
Embodiment 3
[0043] Embodiment 3: Preparation of Glycyrrhetinic Acid-Arginine Injection
[0044] Glycyrrhetinic acid arginine salt (prepared according to Example 1) 5g
[0045] Propylene glycol 150g
[0046] Sodium citrate 10g
[0047] Add water for injection to 1000ml
[0048] Weigh the prescription amount of glycyrrhetinic acid arginine salt, dissolve it in propylene glycol under heating, add sodium citrate and water for injection, adjust the pH to 9.0 with 5% NaOH, add 0.1% activated carbon and stir for 30 minutes, filter, and pack into ampoules Medium, 1ml each, sterilized at 115°C (for 30 minutes, ready to use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com