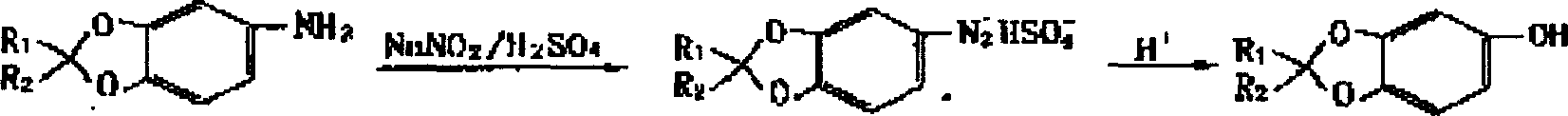Natural foodstuff antioxidant sesamol synthesis method
A synthesis method and antioxidant technology, applied in organic chemistry and other directions, can solve the problems of dark product color, difficulty in refining, product loss, etc., and achieve the effects of cheap raw materials, reduced product coloration, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 13.7 g of piperamine, dissolve it in a solution of 100 ml of water and 27 g of concentrated sulfuric acid, and heat it to clear. Then the temperature was lowered to -5°C, and 30ml of 30% sodium nitrite solution was added for diazotization. Add 0.4 g of urea to decompose unreacted sodium nitrite, and the reaction will reach the end point when the reaction solution no longer turns the potassium iodide test paper blue. The prepared diazonium salt solution was evenly added dropwise to a mixed solution of 60 g of copper sulfate, 15 g of sulfuric acid, 100 ml of water and 300 ml of toluene at 80° C. within 5 hours. After the dropwise addition was completed, the reaction was completed at 80°C for 1 hour. Let stand for layering, separate the lower acid-water layer to be reused in the next reaction. The upper organic layer is washed with a small amount of sodium bicarbonate aqueous solution with heat, then washed with water to neutral, dried with anhydrous sodium sulfate and c...
Embodiment 2
[0026] As in Example 1, the toluene in the hydrolysis reaction mixture was changed to 1,2-dichloroethane, and the hydrolysis was carried out at the reflux temperature, and other conditions remained unchanged. In the end, 10.6 grams of off-white sesamol refined products were obtained, and the yield of sesamol was 76%.
Embodiment 3
[0028] As in Example 1, the toluene in the hydrolysis reaction mixture was changed to xylene, and other conditions remained unchanged. In the end, 11.0 grams of off-white sesamol refined products were obtained, and the yield of sesamol was 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com