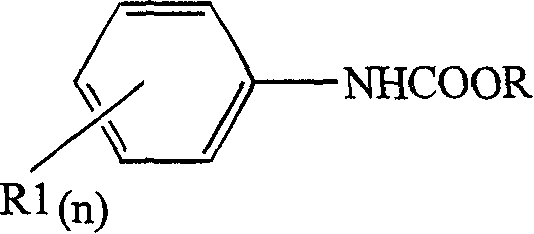
Catalyst for synthesizing diphenyl methane diamino formic ether
A technology of phenylcarbamate and dicarbamate, applied in the field of applied catalysis, can solve the problems of many by-products, unsuitable for large-scale industrial production, and difficulties in separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 2.01g of methyl phenylcarbamate, 0.23g of zirconium oxychloride, 1.05g of 33% formaldehyde solution and 30mL of 27% phosphoric acid solution were charged in a 50mL three-port glass reactor, and the reaction materials and catalyst were all added at one time. The reaction was carried out at 115° C. for 4 hours under normal pressure. After the reaction was completed, the product was obtained by filtering, washing with water and drying. The resulting product and filtrate are analyzed by high-pressure liquid chromatography, and the results are: the conversion rate of methyl phenylcarbamate is 94%, and the yield of 4,4'-diphenylmethanedicarbamate methyl ester is 90.5%, and the selectivity is 96%. %, the yield of methyl 2,4′-diphenylmethanedicarbamate was 4.5%, with almost no detection of methyl 2,2′-diphenylmethanedicarbamate, trinuclear and polynuclear polymethylene polyphenyl The yield of base carbamate is lower.
Embodiment 2
[0020] 3g methyl phenylcarbamate, 1.86g 33% formaldehyde solution, 15mL 18% nitric acid solution, 15mL 30% phosphoric acid solution were charged in the 50mL three-necked glass reactor, and the reaction materials and the catalyzer were added at one time. The reaction was carried out at 100°C under normal pressure for 3 hours. After the reaction was completed, the product was obtained by filtering, washing with water and drying. Gained product and filtrate are analyzed with high-pressure liquid chromatography, and the result is: the conversion rate of methyl phenylcarbamate is 95%, the productive rate of 4,4'-diphenylmethane dicarbamic acid methyl ester is 65%, and selectivity is 68%. %, the yield of methyl 2,4'-diphenylmethanedicarbamate was 3%. In this reaction, no inorganic salt is added as an auxiliary agent, only mixed acid is used as a catalyst, and the yield of methyl 4,4'-diphenylmethanedicarbamate is only 65%.
Embodiment 3
[0022] 2.06g of methyl phenylcarbamate, 0.5g of paraformaldehyde, 0.18g of cobalt acetate and 30mL of 30% phosphoric acid solution were charged in a 50mL three-port glass reactor, and the reaction materials and the catalyst were all added at one time. The reaction was carried out at 105° C. for 5 hours under normal pressure, 30 mL of 30% phosphoric acid solution, the reaction materials and the catalyst were all added at one time. The reaction was carried out at 105° C. for 5 h under normal pressure. After the reaction was completed, the product was obtained by filtering, washing with water and drying. Gained product and filtrate are analyzed with high-pressure liquid chromatography, and the result is: the conversion rate of methyl phenylcarbamate is 92.5%, and the productive rate of 4,4'-diphenylmethane dicarbamate methyl ester is 91%, and selectivity is 98%. %, the yield of methyl 2,4'-diphenylmethanedicarbamate was 2.5%, the selectivity was 2.7%, almost no methyl 2,2'-diphen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com