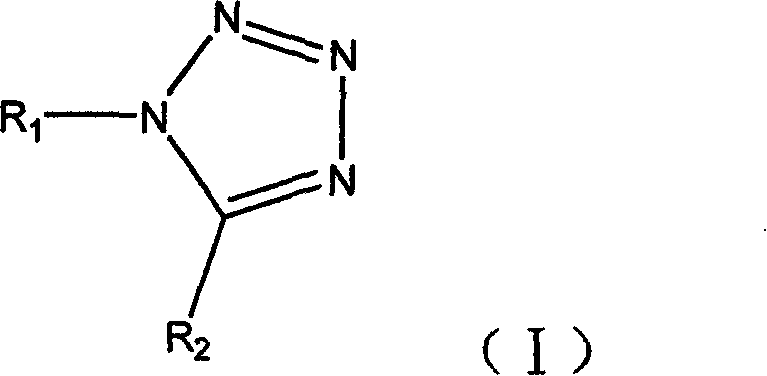
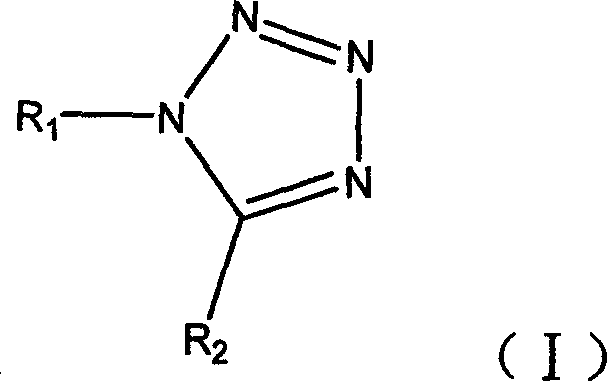
Chemical synthesizing method of tetrazole compound
A technology of chemical synthesis and tetrazolium, applied in the direction of organic chemistry, can solve problems such as environmental pollution, troublesome operation, hidden safety hazards, etc., and achieve the effect of high reaction yield, low production cost, and no three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The feed ratio is amine: orthocarboxylate: metal azide: ytterbium trifluoromethanesulfonate=1: 1: 1: 0.02 (mol ratio), amine is aniline, and orthocarboxylate is triethyl orthoformate, The azide metal salt is sodium azide, and chloroform is used as an organic solvent, and its consumption is 10 times that of aniline.
[0033] In a 150ml four-neck flask equipped with a thermometer, a reflux condenser and mechanical stirring, add aniline 4.56ml (50mmol) at room temperature, sodium azide 3.25g (50mmol), triethyl orthoformate 8.34ml (50mmol), Ytterbium trifluoromethanesulfonate 0.62g (1mmol), dissolve with chloroform 46g, add, be warming up to 60 ℃, and react 3 hours at 60~65 ℃, follow and monitor with HPLC simultaneously (flow velocity: 1.5ml / min, acetonitrile: Water: acetic acid = 20:80:0.05), after the reaction was completed, the solvent was evaporated, and under ice cooling, 20ml of ice water was slowly added, stirred continuously for 10min, and extracted three times with...
Embodiment 2
[0037] The feed ratio is amine: orthocarboxylate: metal azide: ytterbium trifluoromethanesulfonate=1: 1: 1: 0.02 (mol ratio), amine is aniline, and orthocarboxylate is triethyl orthoformate, The azide metal salt is sodium azide, and chloroform is used as an organic solvent, and its consumption is 8 times that of aniline.
[0038] Other operations are the same as in Example 1, the product yield is 81.1%, the purity is 99.6%, the melting point is 64.7-65.1°C, and the recovery rate of ytterbium trifluoromethanesulfonate is 94.2%.
Embodiment 3
[0040] The feed ratio is amine: orthocarboxylate: metal azide: ytterbium trifluoromethanesulfonate=1: 1: 1: 0.02 (mol ratio), amine is aniline, and orthocarboxylate is triethyl orthoformate, The azide metal salt is sodium azide, and chloroform is used as an organic solvent, and its consumption is 5 times that of aniline.
[0041] Other operations are the same as in Example 1, the product yield is 80.5%, the purity is 99.7%, the melting point is 64.5-65.0°C, and the recovery rate of ytterbium trifluoromethanesulfonate is 95.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com