Modified butanediol ester poly succinic acid and synthetic method
A technology of polybutylene succinate and butanediol, which is applied in the field of maleopimaric anhydride copolymerized modified polybutylene succinate and its synthesis, can solve the impact of biodegradation performance and the toxic and side effects of degradation products To achieve the effect of improving crystallization behavior, avoiding toxic and side effects, and reducing crystallization temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 0.1mol of maleopimaric anhydride (RMA), 0.1mol of glycine, and 150ml of N,N-dimethylformamide (DMF) into a four-neck flask equipped with a thermometer, stirring, and nitrogen protection device, and stir at room temperature for 8 hours , heated to 130°C and stirred for 4 hours, cooled to room temperature, and turned into a yellow-brown solid, added 200ml of water, stirred, and a yellow precipitate formed. Filtration, washing, vacuum drying for 3 hours, repeated recrystallization with DMF and water, the final product became a white powdery solid, placed in a vacuum oven at 90° C. for 10 hours of vacuum drying to obtain maleopimaric acid imide dicarboxylic acid ( RMID), spare, 56% yield.
[0041]Take 0.05 mol of maleopimaric acid imide dicarboxylic acid prepared in the previous step and add excess 1,4-butanediol into a four-neck flask equipped with stirring, heating, and nitrogen protection device, at 230-300°C The mixture of zinc acetate and antimony trioxide is used...
Embodiment 2
[0044] Add 0.1 mol of maleopimaric anhydride (RMA), 0.1 mol of 6-aminocaproic acid, and 150 ml of N, N-dimethylformamide (DMF) into a four-neck flask equipped with a thermometer, stirring, and nitrogen protection device, and stir at room temperature After 18 hours, the temperature was raised to 140° C. and stirred for 4 hours, cooled to room temperature, and a yellow-brown solid was formed. Added 200 ml of water, stirred, and a yellow precipitate formed. Filtration, washing, vacuum drying for 5 hours, repeated recrystallization with DMF and water, the final product was a white powdery solid, placed in a vacuum drying oven at 90°C for 10 hours in vacuum to obtain maleopimaric acid imide dicarboxylic acid, Standby, yield 70%.
[0045] Take 0.05 mol of maleopimaric acid imide dicarboxylic acid prepared in the previous step and add excess 1,4-butanediol into a four-neck flask equipped with stirring, heating, and nitrogen protection device, at 230-300°C Using antimony trioxide as ...
Embodiment 3
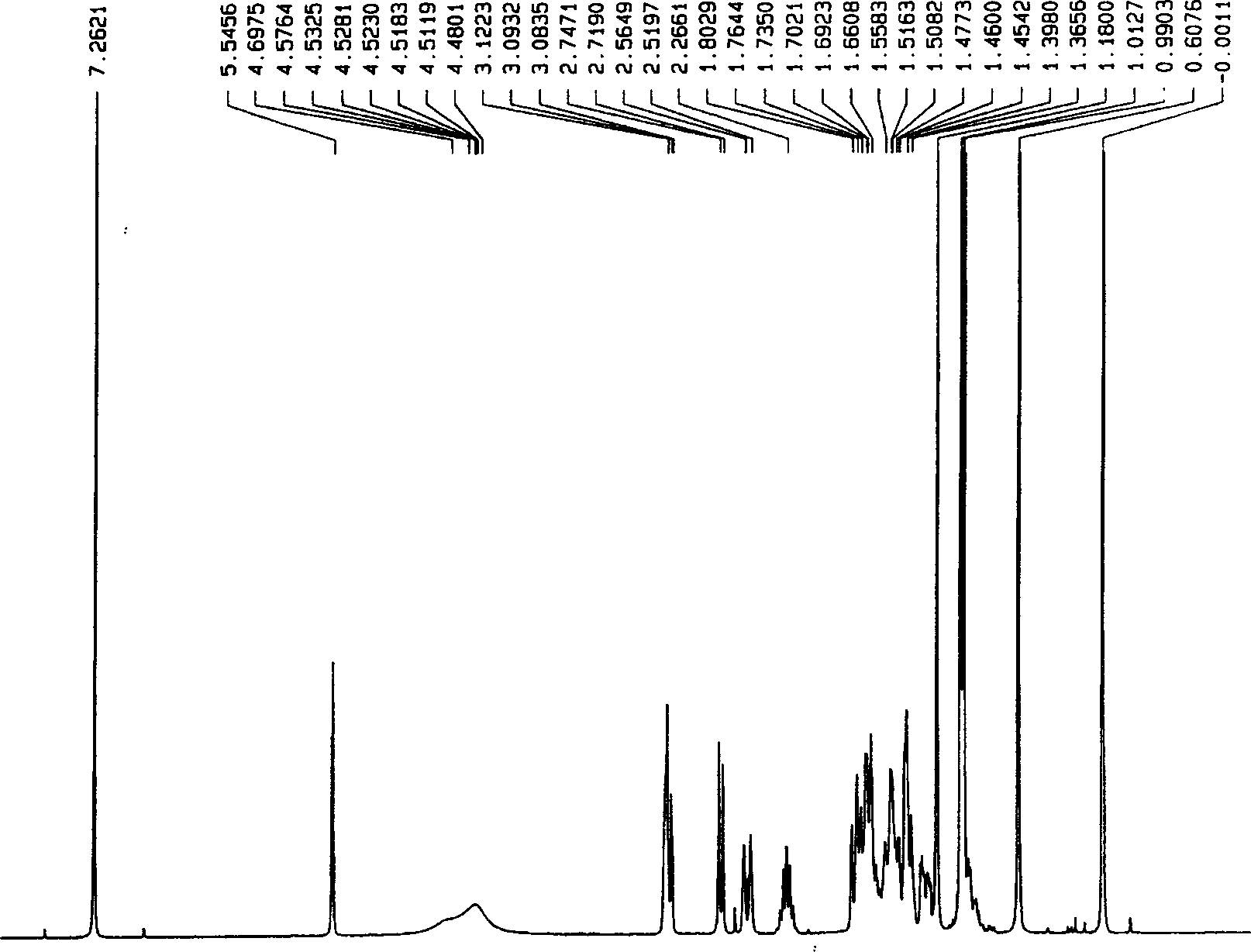
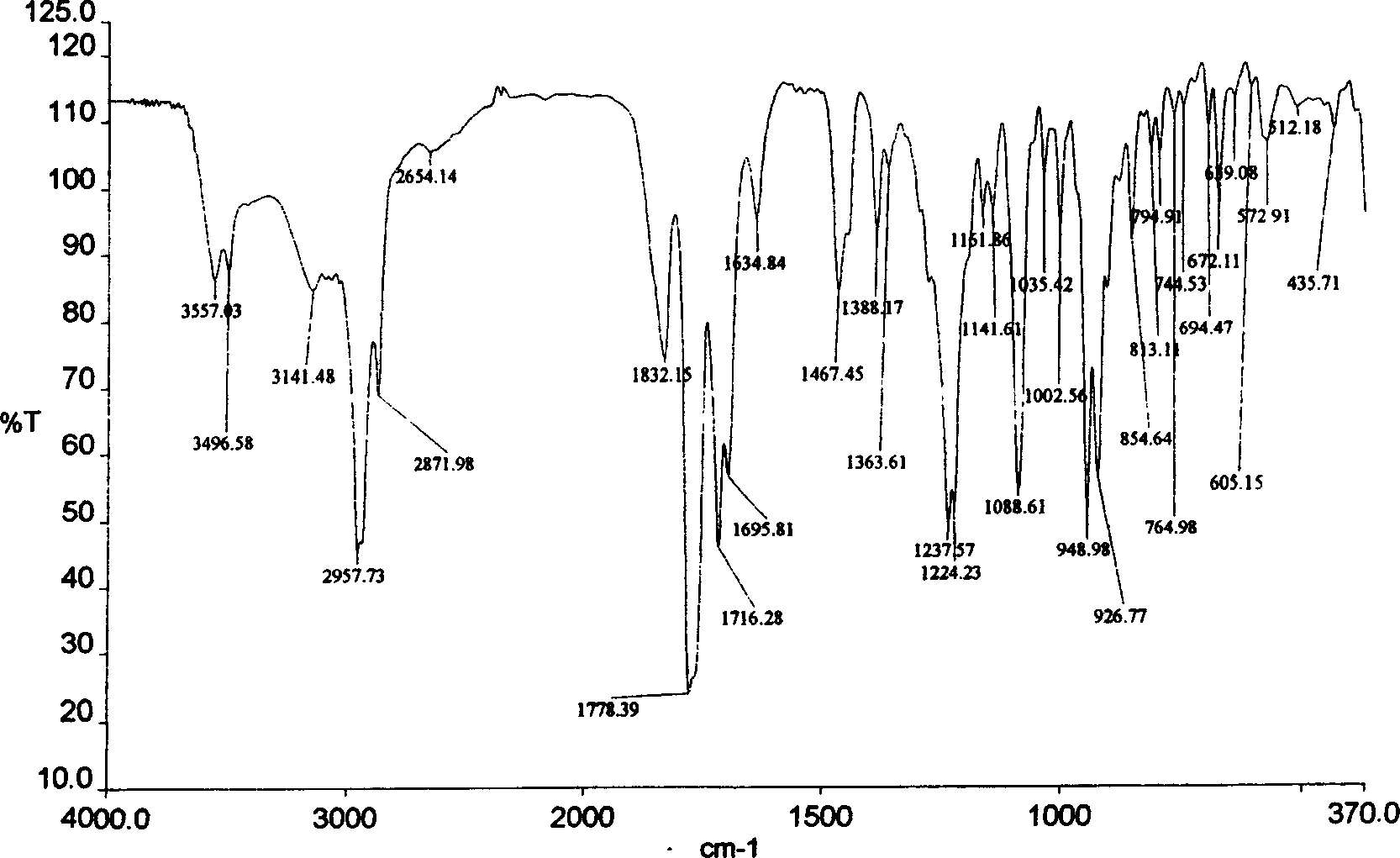
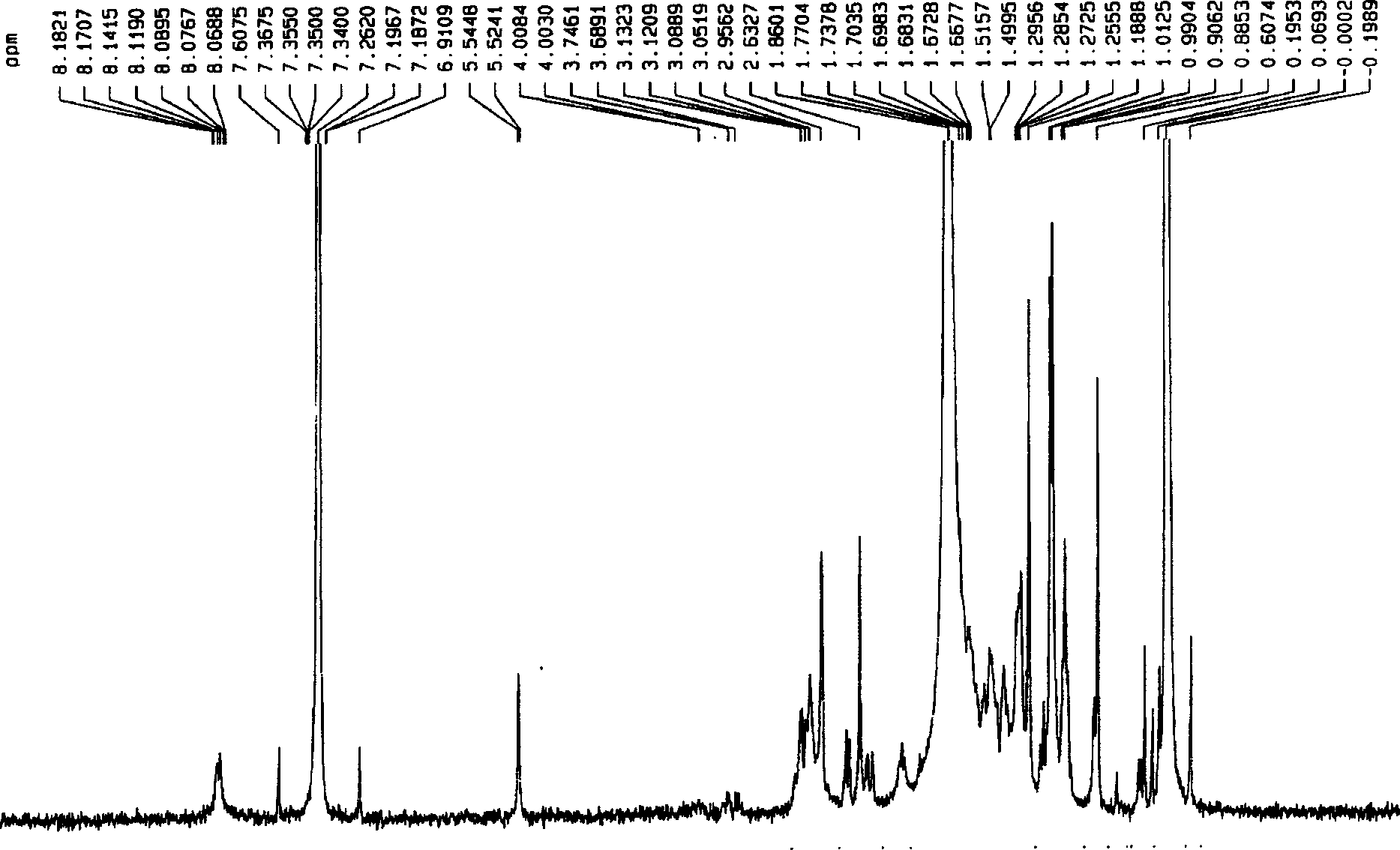
[0048] Add 0.1mol of maleopimaric anhydride (RMA), 0.1mol of p-aminobenzoic acid, and 150ml of N,N-dimethylformamide (DMF) into a four-neck flask equipped with a thermometer, stirring, and nitrogen protection device, and stir at room temperature for 3 After 1 hour, the temperature was raised to 150° C. and stirred for 2 hours, cooled to room temperature, and a yellow-brown solid was formed. Added 200 ml of water, stirred, and a yellow precipitate formed. Filter and wash, vacuum dry for 3 hours, DMF and water recrystallize repeatedly, the final product becomes a white powdery solid, put it in a vacuum drying oven at 90°C and vacuum dry for 10 hours to obtain maleopimaric acid imide dicarboxylic acid (RMID) , standby, yield 86%. See Figure 1~4 .
[0049] Take 0.05 mol of maleopimaric acid imide dicarboxylic acid prepared in the previous step and add excess 1,4-butanediol into a four-neck flask equipped with stirring, heating, and nitrogen protection device, at 230-300°C A mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com