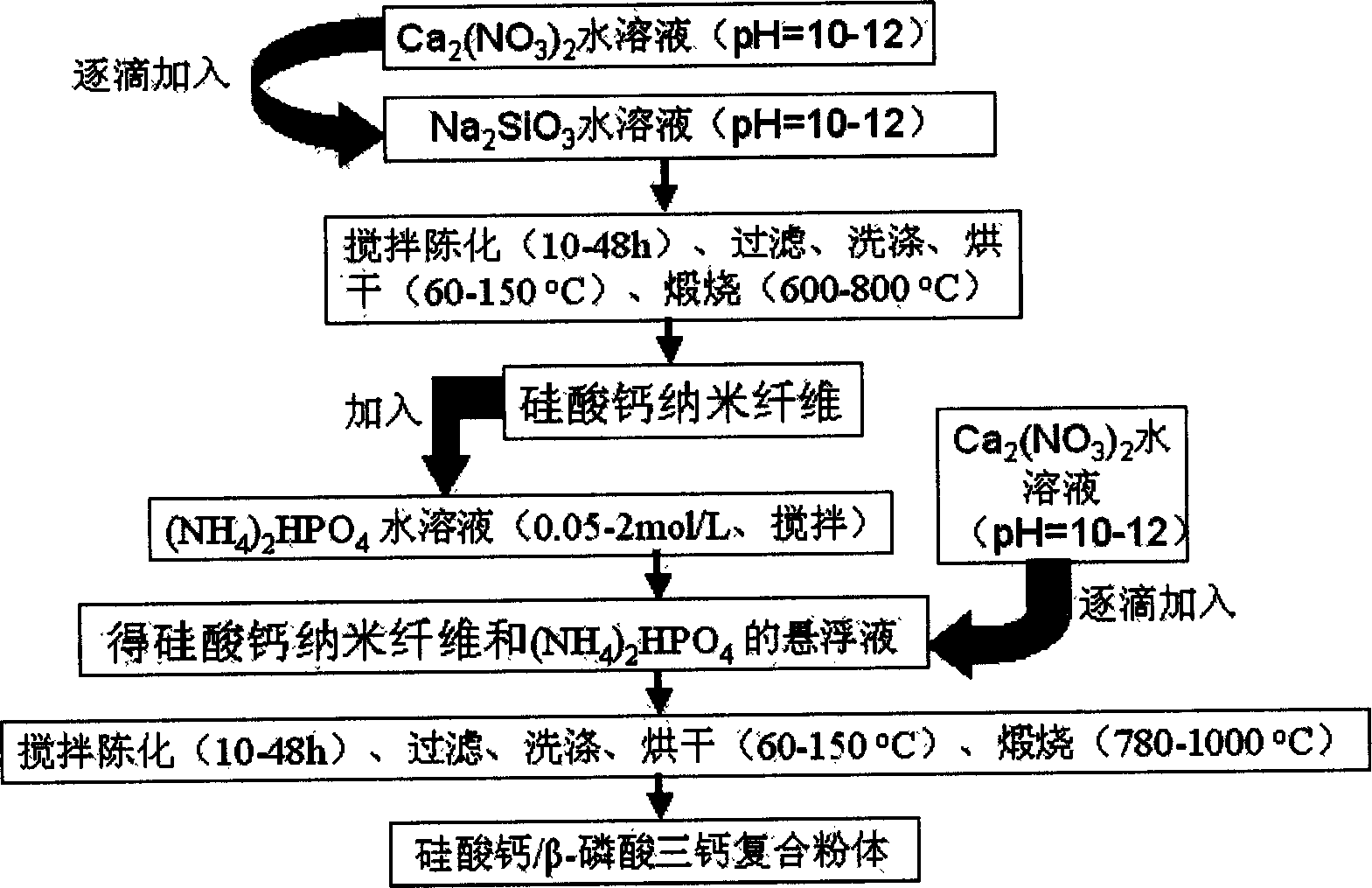
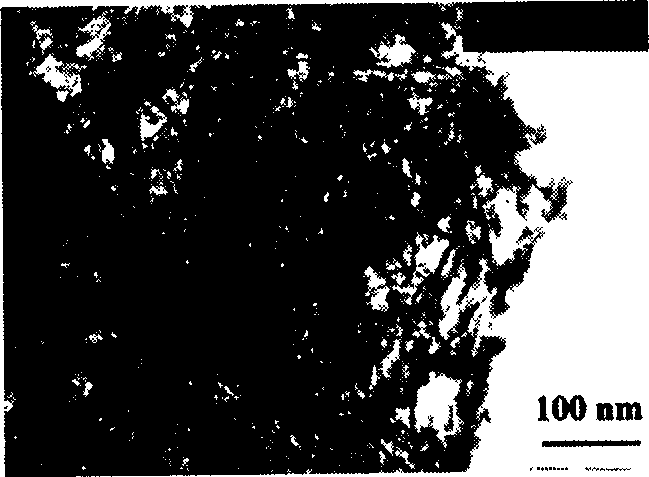
Method for preparing calcium silicate/beta- tricalcium phosphate composite powder by two-step chemical precipitation method
A technology of tricalcium phosphate nanometer and composite powder, which is applied in the field of biomedical materials, can solve the problems of cumbersome washing, high temperature, complexity, etc., and achieves the effects of simple and easy preparation process, small grain size, and controllable composite ratio.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take 40.72 grams of Ca(NO 3 ) 2 4H 2 O was dissolved in 1000 mL of deionized water and adjusted to pH = 11 with 1:1 ammonia water, and 49.00 g of Na 2 SiO 3 9H 2 Dissolve O in 1000 mL deionized water to obtain Na 2 SiO 3 aqueous solution. The above Ca(NO 3 ) 2 The solution was added dropwise to Na 2 SiO 3 In the solution, use 1:1 ammonia solution to keep the pH value of the reaction system at 11 during the addition process, continue stirring for 24 hours after addition, filter, wash with deionized water and absolute ethanol, filter dry, and dry at 80°C for 12 hours A dry powder is obtained, and the dry powder is calcined at 750° C. for 2 hours to obtain a white calcium silicate powder. Disperse the calcium silicate powder prepared above in 1000mL and dissolve 17.04 grams of (NH 4 ) 2 HPO 4 1000mL of pH 11.0 and 45.71 grams of Ca(NO 3 ) 2 4H 2 O aqueous solution was added dropwise to the above-mentioned suspension, and the pH value of the reaction system...
Embodiment 2
[0024] Take 49.60 grams of Ca(NO 3 ) 2 4H 2 O was dissolved in 1500 mL of deionized water, and adjusted to pH = 10.5 with aqueous sodium hydroxide solution, and 59.39 g of Na 2 SiO 3 9H 2 Dissolve O in 1000 mL deionized water to obtain Na 2 SiO 3 aqueous solution. The above Ca(NO 3 ) 2 solution added to Na 2 SiO 3 In the solution, use sodium hydroxide aqueous solution to keep the pH value of the reaction system at 10.5 during the addition process, continue to stir for 24 hours after addition, filter, wash with deionized water and absolute ethanol, filter dry, and dry at 80°C for 12 hours to obtain a dry powder body, the dry powder was calcined at 700°C for 2 hours to obtain white calcium silicate powder. The calcium silicate powder that above-mentioned preparation obtains is dispersed in 1000mL and is dissolved with 48.44 grams (NH 4 ) 2 HPO 4 1000mL of pH 10.5 and 129.93 grams of Ca(NO 3 ) 2 4H 2 O aqueous solution was added dropwise to the above suspension. ...
Embodiment 3
[0026] Take 86.80 grams of Ca(NO 3 ) 2 4H 2 O was dissolved in 1000 mL deionized water, and adjusted to pH = 11 with 1:1 ammonia water, and 103.94 g of Na 2 SiO 3 9H 2 Dissolve O in 1000 mL deionized water to obtain Na 2 SiO 3 aqueous solution. The above Ca(NO 3 ) 2 The solution was added dropwise to Na 2 SiO 3 In the solution, use 1:1 ammonia solution to keep the pH value of the reaction system at 11 during the addition process, continue stirring for 24 hours after addition, filter, wash with deionized water and absolute ethanol, filter dry, and dry at 80°C for 12 hours A dry powder is obtained, and the dry powder is calcined at 750° C. for 2 hours to obtain a white calcium silicate powder. The calcium silicate powder prepared above was strongly dispersed in 1000mL dissolved with 15.57 grams of (NH 4 ) 2 HPO 4 1000mL of pH 11.0 and 41.76 grams of Ca(NO 3 ) 2 4H 2 O aqueous solution was added dropwise to the above-mentioned suspension, and the pH value of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com