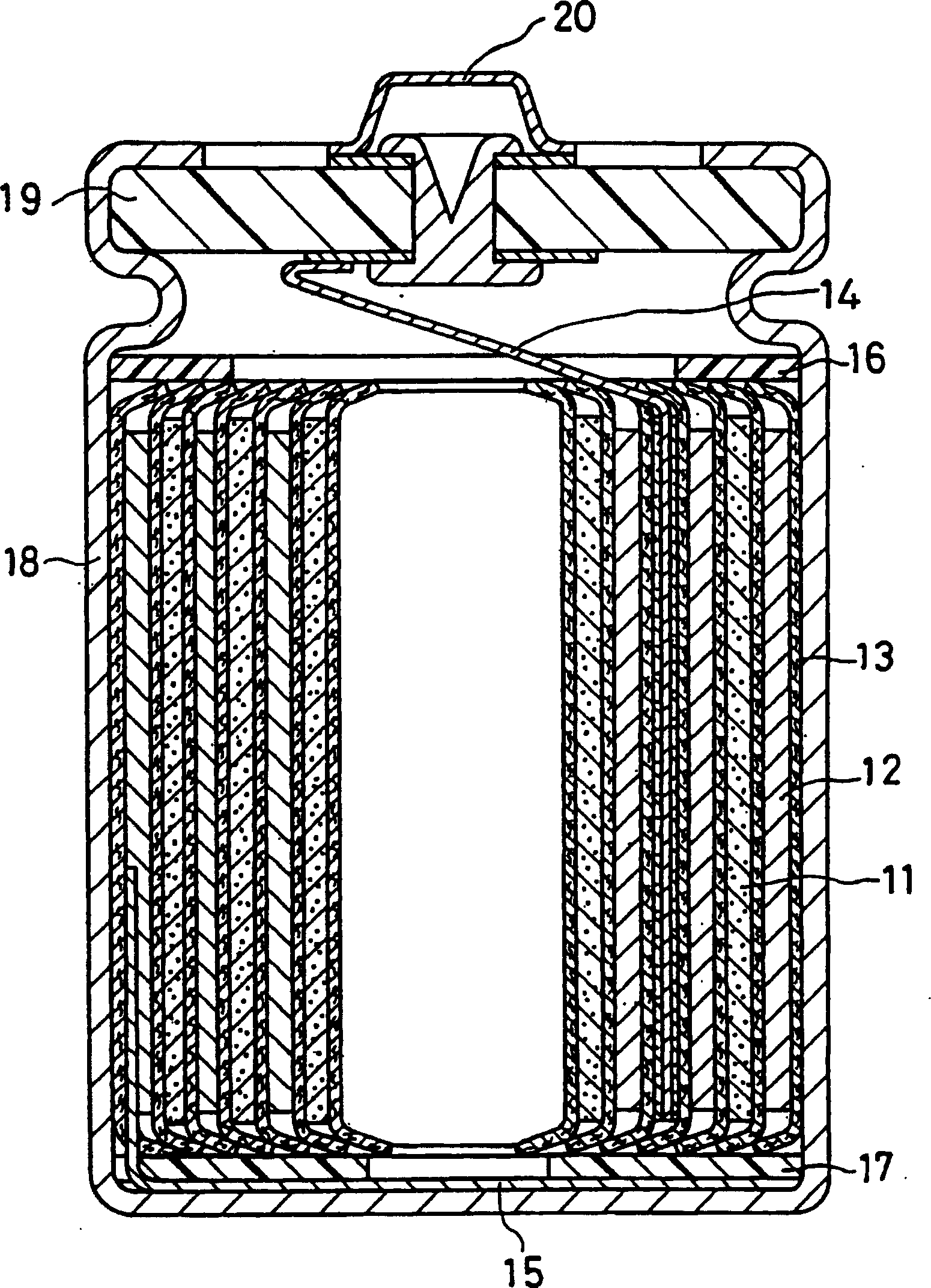
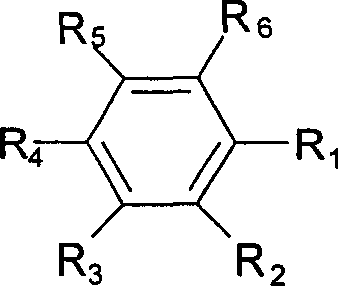
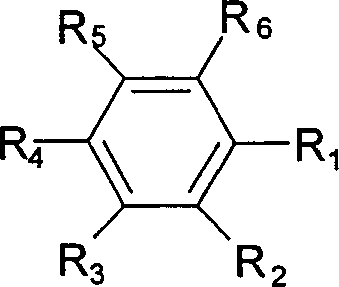
Non-aqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve problems such as no effective solutions, achieve good characteristics, avoid degradation, and suppress side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (i) Preparation of non-aqueous electrolyte
[0048] Dissolve LiPF at a concentration of 1.0 mol / L in a mixed solvent of ethylene carbonate (EC) and methyl ethyl carbonate (EMC) (volume ratio 1:4) 6 . To the obtained solution, 5 parts by weight of fluorobenzene (FB) was added per 100 parts by weight of the nonaqueous solvent to obtain a nonaqueous electrolyte.
[0049] (ii) Fabrication of the positive electrode
[0050] will be mixed with 85 parts by weight of positive electrode active material LiNi 0.8 co 0.2 o 2 The anode mixture of the conductive material acetylene black of powder, 10 parts by weight and the binder polyvinylidene fluoride of 5 parts by weight is dispersed in dehydrated N-methyl-2-pyrrolidone (NMP), thereby preparing the anode mixture material pulp. This slurry was coated on both surfaces of a positive electrode current collector made of aluminum foil, dried, and then rolled to obtain a positive electrode.
[0051] (iii) Preparation of negative ...
Embodiment 2
[0070] A battery similar to that of Example 1 was produced except that the nickel-containing lithium composite oxide having the composition shown in Table 2 was used as the positive electrode active material, and the same evaluation was performed. The results are shown in Table 2.
[0071] positive active material
Fluorobenzene (parts by weight)
Capacity maintenance rate (%)
Example 2
LiNi 0.005 co 0.995 o 2
5
80.2
LiNi 0.05 co 0.95 o 2
5
80.9
LiNi 0.1 co 0.9 o 2
5
83.0
LiNi 0.3 co 0.7 o 2 x
5
85.1
LiNi 0.5 co 0.5 o 2
5
85.3
LiNi 0.7 co 0.3 o 2
5
86.0
LiNi 0.8 co 0.2 o 2
5
86.5
LiNi 0.9 co 0.1 o 2
5
86.1
LiNiO 2
5
81.3
LiNi 0.8 co 0.15 Al 0.05 o 2
5
88.2
LiNi 0.8 co 0.15 Sr 0.05 o 2
5
8...
Embodiment 3
[0076] Except for using the compounds shown in Table 3 as the fluorine atom-containing aromatic compound contained in the non-aqueous electrolyte, the same batteries as in Example 1 were fabricated and evaluated in the same manner. The results are shown in Table 3.
[0077] Aromatic compounds containing fluorine atoms
Capacity maintenance rate (%)
Example 3
86.5
1,2-Difluorobenzene
85.1
1,2,3-Trifluorobenzene
85.0
1,2,3,4-Tetrafluorobenzene
84.8
Pentafluorobenzene
84.5
84.2
2-fluorotoluene
82.7
α,α,α-trifluorotoluene
80.3
3-fluoro-o-xylene
81.4
80.8
2-fluorostyrene
82.2
4-fluorostyrene
82.9
[0078] As can be seen from Table 3, regardless of the type of aromatic compound containing fluorine atoms, by combining the positive electr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com