Charles ketone oxime and its composition , preparation method and uses
A composition and technology of dimethyl chalcone are applied in the directions of oxime preparation, drug combination, amine active ingredients, etc., which can solve the problems of poor solubility and low oral bioavailability of chalcone, and achieve good solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of the compound of formula (1)
[0021] Take 100mg of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone and 100mg of hydroxylamine hydrochloride, add 7mL of pyridine to dissolve, heat and reflux for 2 hours, pour the reaction solution into In 50mL of 1mol / L hydrochloric acid aqueous solution, filter to collect the precipitate, dissolve it with ethyl acetate, transfer the ethyl acetate solution into a separating funnel, wash with water three times, concentrate under reduced pressure to dryness, put on a silica gel column, and dissolve with petroleum ether-acetone (Volume ratio 6:1) was eluted and isolated to obtain the product.
Embodiment 2
[0022] Embodiment 2: the identification of embodiment 1 product
[0023] Yellow powdery solid. Positive ion HRTOFMS (m / z): 314.1398[C 18 h 19 NO 4 +H] + (calculated value: 314.1392). Positive ion ESIMS: 352[M+K] + , 336[M+Na] + , 314[M+H] + , 296[M-H 2 O+H] + , 280, 220, 208, 193, 103. 1 H NMR (400MHz, acetone-d 6 ): 7.42 (2H, d, J = 8.0Hz, H-2 and H-6), 7.30 (2H, t, J = 8.0Hz, H-3 and H-5), 7.22 (1H, t, J = 8.0Hz, H-4), 7.05(1H, d, J=16Hz, H-β), 6.41(1H, d, J=16Hz, H-α), 3.53(3H, s, OCH3), 2.11(3H ,s,CH 3 ), 2.09 (3H, s, CH 3 ); 13 C NMR (100MHz, acetone-d 6 ): 155.8(C×2), 155.1(C), 151.2(C), 137.6(C), 134.6(CH), 129.4(CH×2), 128.8(CH), 127.5(CH×2), 127.0( CH), 110.0(C), 108.4(C), 107.0(C), 61.0(OCH 3 ), 9.3 (CH 3 ), 9.2 (CH 3 ). By analysis, it is confirmed that the product obtained in Example 1 is a compound of formula (1), i.e. 2′, 4′-dihydroxy-6′-methoxyl group-3′, 5′-dimethylchalcone oxime (DMDO ).
Embodiment 3
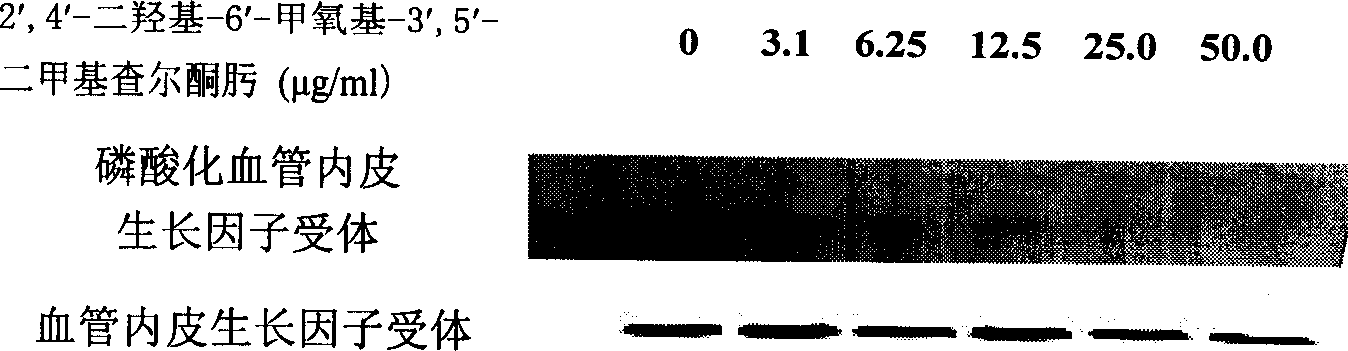
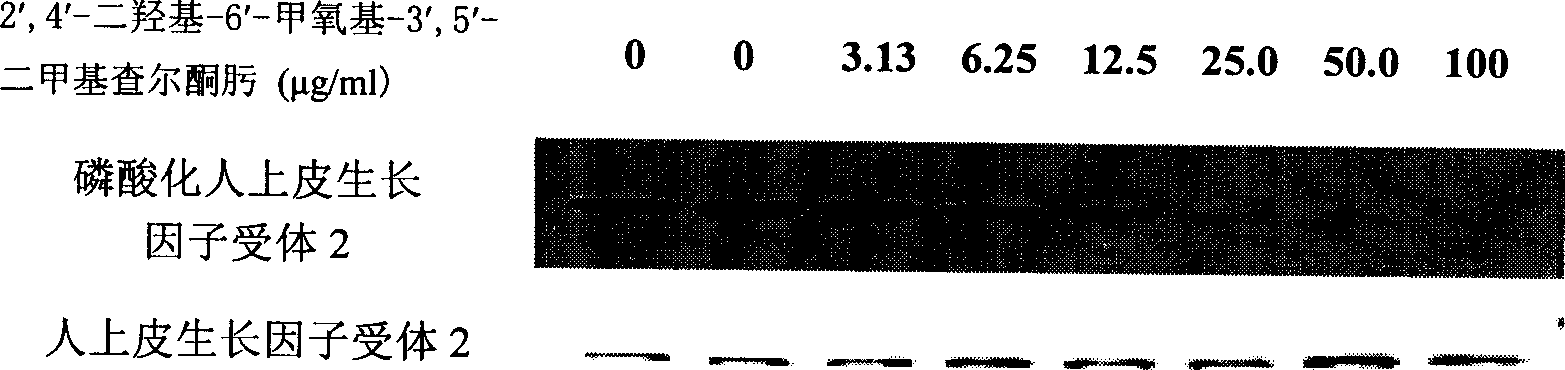
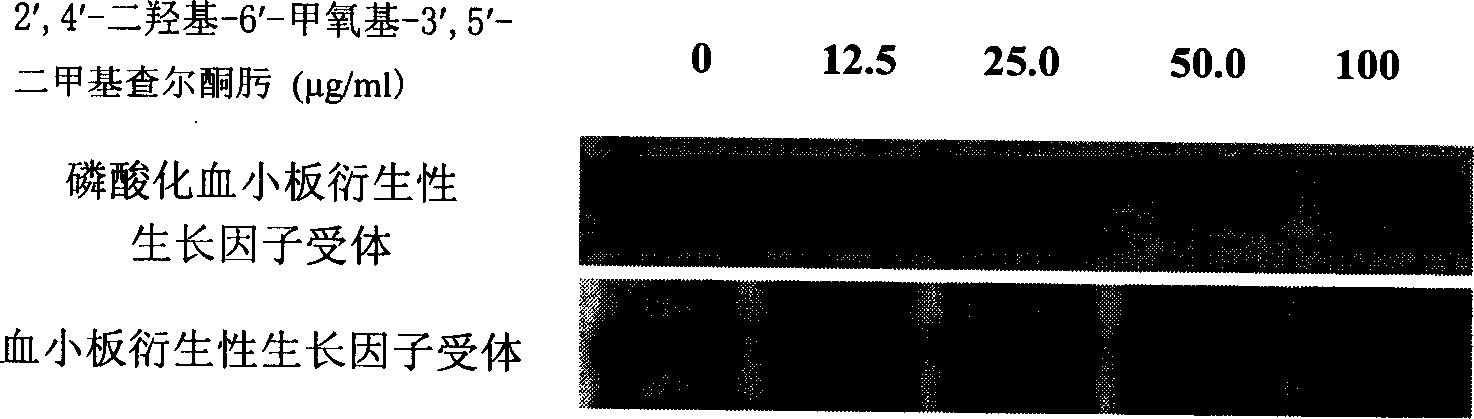
[0024] Example 3: Inhibitory Effect of DMDO on Phosphorylation of Receptor Tyrosine Kinases
[0025] Take ECV304 (VEGFR2 or KDR), MDA-MB-453 (HER2), C6 (PDGFR) cells in the logarithmic growth phase, and make 5×10 5 / mL, then add it to a 12-well plate, add 1mL to each well. On the second day, the culture solution was aspirated, and serum-free culture solution was added, and then different concentrations of DMDO were added for treatment for 30 minutes, and anti-KDR, anti-HER2, and anti-PDGFR antibodies were used for immunoprecipitation, and then anti-phosphotyrosine Antibody Western blot was used to detect the phosphorylation levels of KDR, HER2 and PDGFR. see attached results Figure 1~3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com