Indole-3-formic acid purification process
A technology for indole and formic acid, which is applied in the field of purification technology of indole-3-carboxylic acid, can solve the problems of color deterioration, high residue and high impurity content, and achieves the effects of being beneficial to industrialized production, easy to obtain raw materials, and easy to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Preparation of indole-3-carboxylic acid potassium salt solution
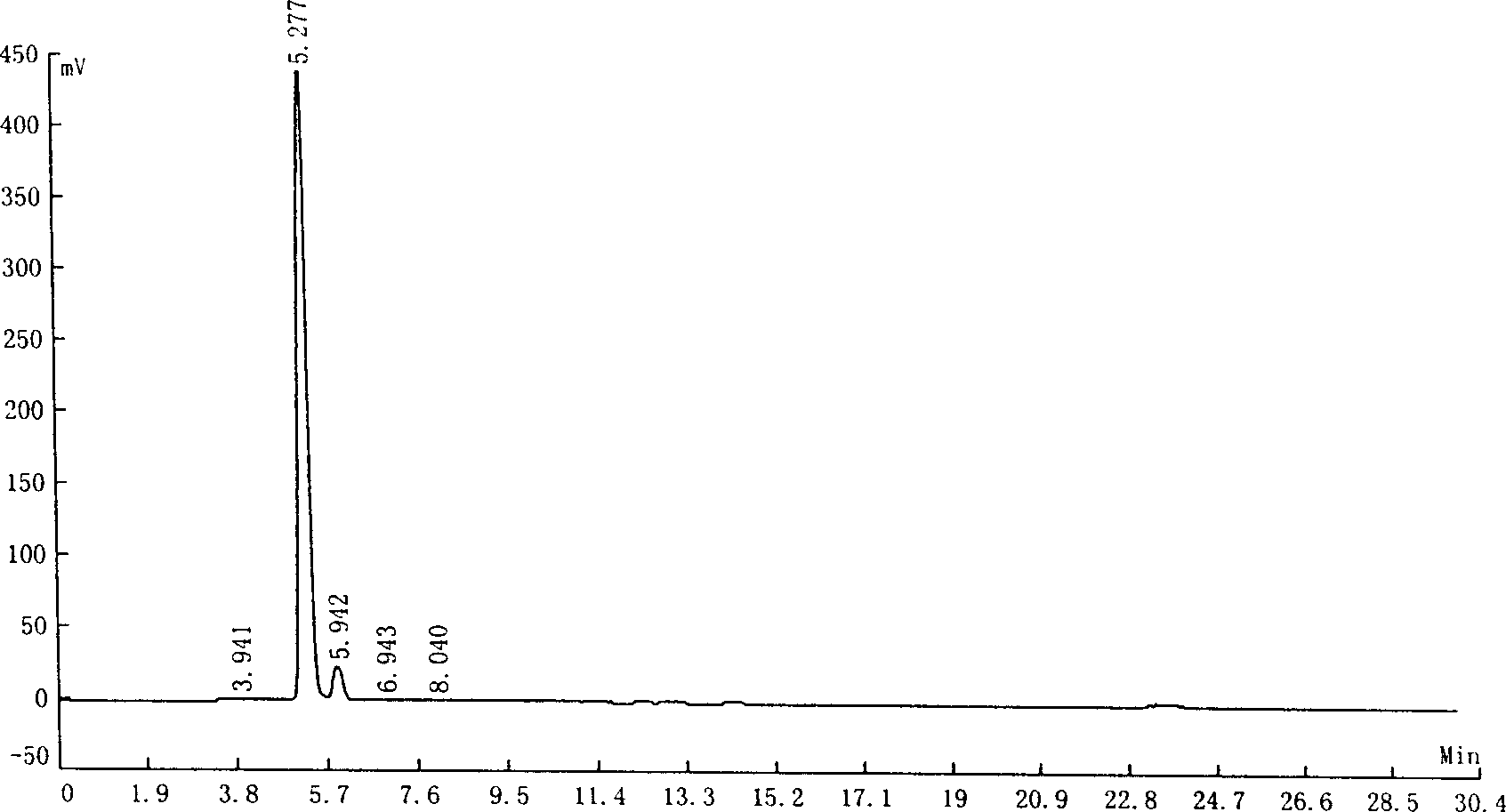
[0038] Add water (200 milliliters), potassium hydroxide (20.0 grams, 0.5 moles), indole-3-formaldehyde (14.5 grams, 0.1 moles) in the 500 milliliters three-neck reaction flasks with mechanical stirrer and reflux condenser, oxidation Silver (4.8 grams, 0.025 mole), be heated to 60 ℃, drip concentration and be 30% hydrogen peroxide aqueous solution (22.5 grams, 0.2 mole) reaction 5 hours, cool, filter (remove silver oxide and unreacted indole- 3-formaldehyde), to obtain 255 grams of aqueous solution of the potassium salt of indole-3-formic acid.
[0039] According to liquid chromatography analysis, the content of indole-3-carboxylic acid was 94.32%, and that of indole-3-carbaldehyde was 5.43%.
[0040] The test conditions of liquid chromatography are as follows:
[0041] Chromatograph: LC-10AT
[0042] Chromatographic column: 250×4.6mm SS EXSIL ODS 5μm
[0043] Mobile phase: methanol / water=6:4
[0044]...
Embodiment 1
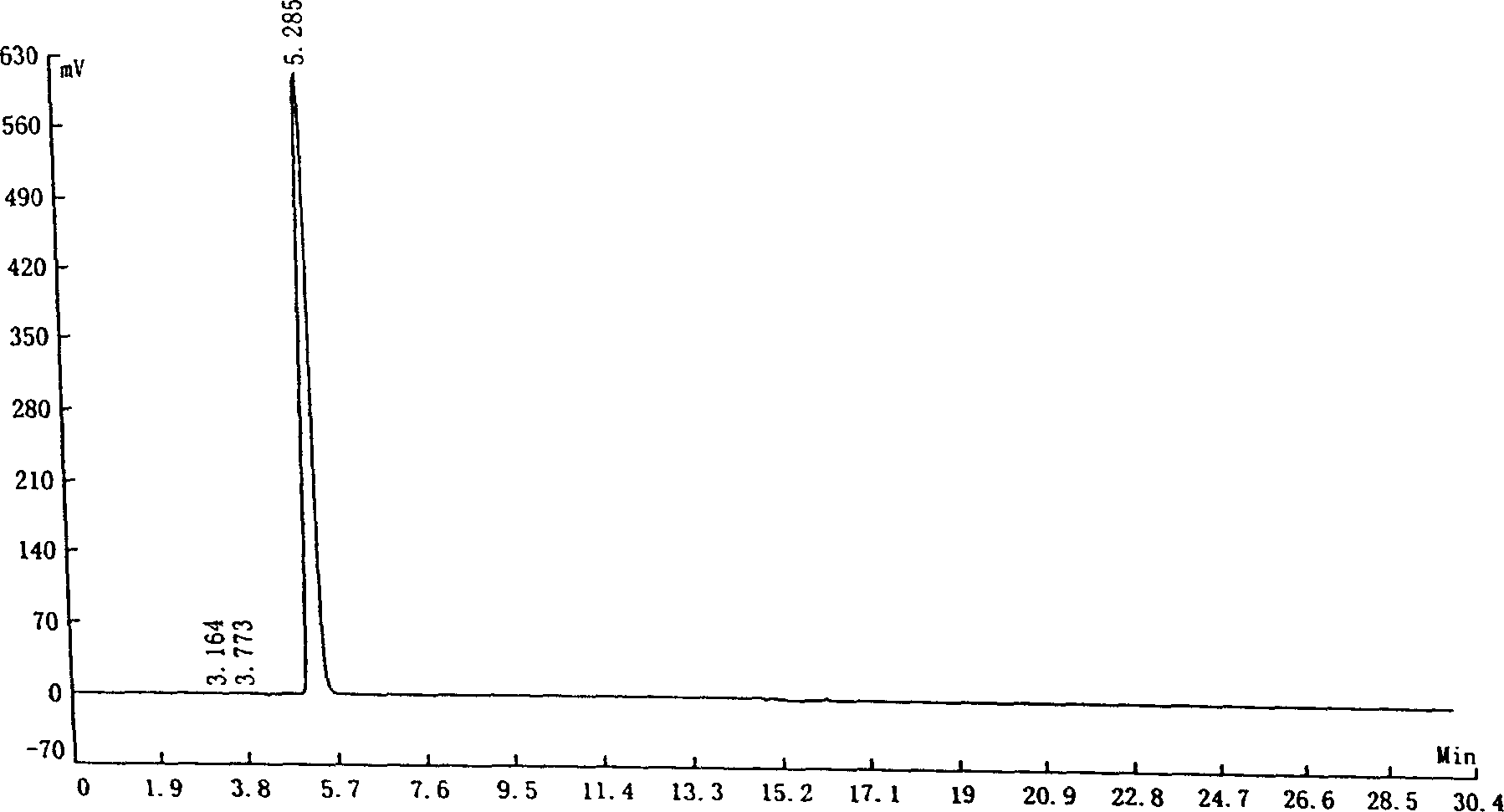
[0049] The potassium salt solution of indole-3-carboxylic acid (100 grams, concentration of indole-3-carboxylic acid is 5.4%) prepared according to Reference Example 1 was warmed up to 55-60°C, and methyl butyl ketone (2 grams) was added , keep warm for 1 hour, cool down to room temperature, filter, add concentrated hydrochloric acid to the filtrate, adjust pH = 4-5, filter the precipitate, and dry to obtain 5.1 g of light yellow indole-3-carboxylic acid.
[0050] Liquid chromatography analysis: indole-3-carboxylic acid content: 99.54%; indole-3-carbaldehyde content: 0.07%.
Embodiment 2
[0052] The potassium salt solution of indole-3-carboxylic acid (100 grams, the concentration of indole-3-carboxylic acid is 5.4%) prepared according to Reference Example 1 was warmed up to 55-60° C., and methyl isopropyl ketone (2 grams ), heat preservation for 1 hour, cool to room temperature, filter, add concentrated hydrochloric acid to the filtrate, adjust pH=4~5, filter the precipitate, dry to obtain 4.9 grams of light yellow indole-3-carboxylic acid.
[0053] Liquid chromatography analysis: indole-3-carboxylic acid content: 99.71%; indole-3-carbaldehyde content: 0.08%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com