Prepn process of 4-amino-2-trifluoromethyl benzonitrile
A technology of trifluoromethyl benzonitrile and trifluoromethyl fluorobenzene, applied in the field of preparing 4-amino-2-trifluoromethyl benzonitrile, can solve the problem of high production cost, many raw materials, many by-products, etc. problems, to achieve the effect of low production cost, simple process and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
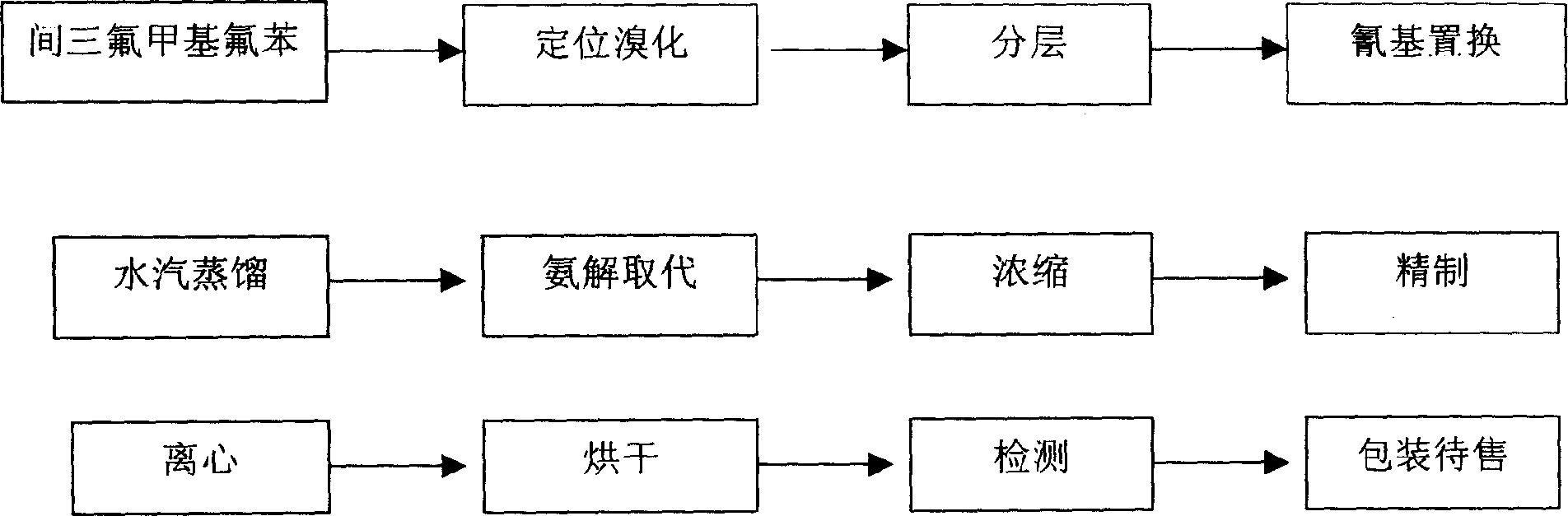
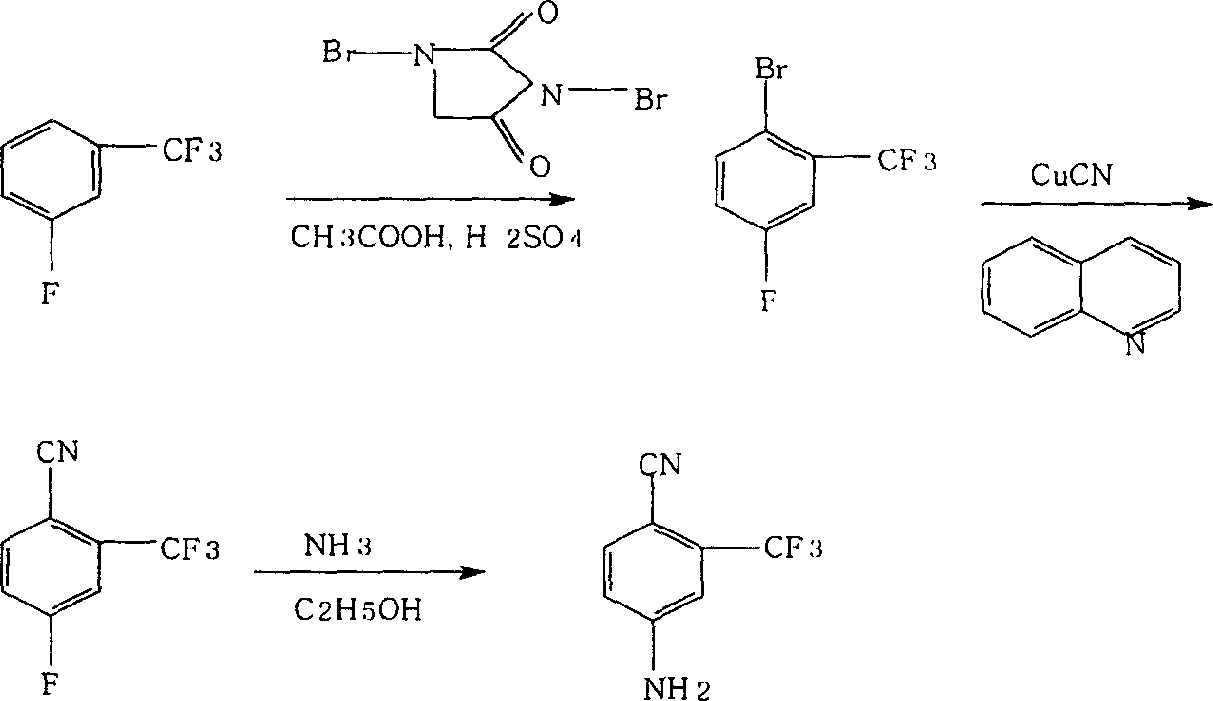
[0017] The first step of positioning bromination: put 200kg m-trifluoromethylfluorobenzene, 80kg glacial acetic acid, and 35kg concentrated sulfuric acid into the reaction pot, mix and stir, heat up and reflux, add 188kg dibromohydantoin in batches, react for 6 hours, and store on ice Wash with water to obtain 260kg of 4-fluoro-2-trifluoromethyl bromide, with a content of more than 98%;
[0018] The second step of cyano group replacement: Take 250kg quinoline and 106kg cuprous cyanide and add dropwise the 4-fluoro-2-trifluoromethylbromobenzene obtained in the first step under the state of stirring and reflux. Distill 200kg of 4-fluoro-2-trifluoromethylbenzonitrile;
[0019] The third step of ammonolysis and substitution: dissolve the 4-fluoro-2-trifluoromethylbenzonitrile obtained in the second step in 350 kg of ethanol, feed in 27 kg of liquid ammonia, and heat up to 120°C for 8 hours to obtain 4- Amino-2-trifluoromethylbenzonitrile crude product 185kg, finally obtains finis...
Embodiment 2
[0022] The first step of positioning bromination: put 250kg of m-trifluoromethylfluorobenzene, 100kg of glacial acetic acid, and 44kg of concentrated sulfuric acid into the reaction pot, mix and stir, heat up and reflux, add 235kg of dibromohydantoin in batches, react for 6.5 hours, and store on ice Wash with decomposed water to obtain 4-fluoro-2-trifluoromethyl bromobenzene;
[0023] The second step of cyano replacement: take 310kg of quinoline and 132kg of cuprous cyanide and add dropwise the 4-fluoro-2-trifluoromethylbromobenzene obtained in the first step under the state of stirring and reflux. 4-fluoro-2-trifluoromethylbenzonitrile is distilled off;
[0024] The third step of ammonolysis and substitution: dissolve the 4-fluoro-2-trifluoromethylbenzonitrile obtained in the second step in 440 kg of ethanol, pass through 34 kg of liquid ammonia, and heat up to 122 ° C for 10 hours to obtain 4- Amino-2-trifluoromethylbenzonitrile crude product 211kg, finally obtains finished...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com